2022 Annual Meeting Accepted Abstracts
*Indicates selected for podium presentation
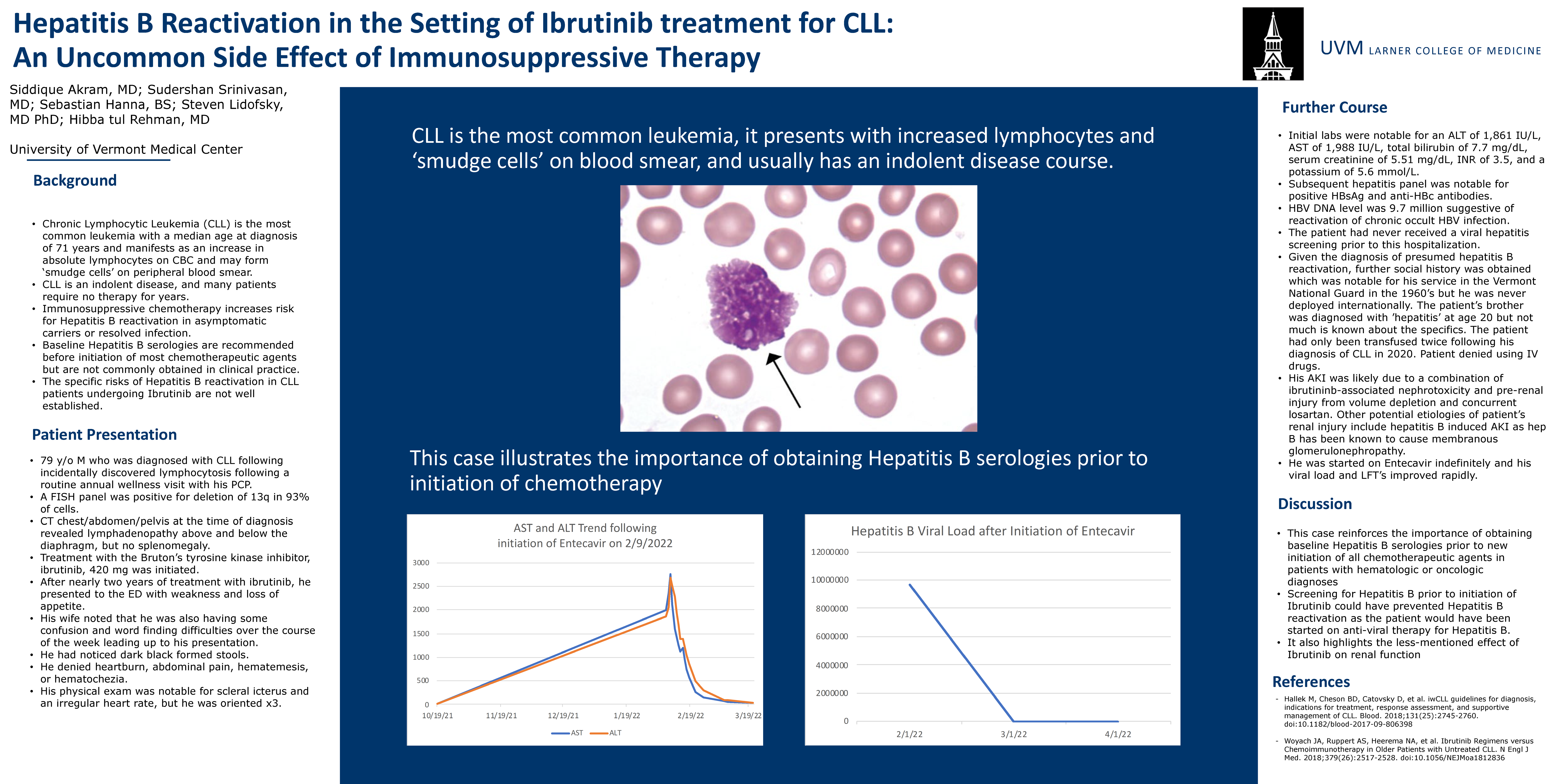
Hepatitis B Reactivation in the setting of Ibrutinib treatment for CLL: an uncommon side effect of immunosuppressive therapy
Siddique Akram, MD, Sudershan Srinivasan, MD; Sebastian Hanna; Hibba Rehman, MD; Steve Lidofsky, MD PhD, University of Vermont Medical Center & Larner College of Medicine at the University of Vermont
Background
Immunosuppressive chemotherapy increases risk for Hepatitis B reactivation in asymptomatic carriers or resolved infection. Baseline Hepatitis B serologies are recommended before initiation of most chemotherapeutic agents but are not commonly obtained in clinical practice. The specific risks of Hep B reactivation in CLL patients undergoing Ibrutinib therapy are not well established.
Methods
Here we present a 79 y-o man diagnosed with CLL following incidentally discovered lymphocytosis during routine health examination. A FISH panel was positive for deletion of 13q in 93% of cells and treatment with ibrutinib 420 mg daily was initiated. After nearly two years of treatment with ibrutinib, he presented to the ED with weakness and loss of appetite.
Results
Labs were significant for an ALT of 1,861 IU/L, AST of 1,988 IU/L, total bilirubin of 7.7 mg/dL, serum creatinine of 5.51 mg/dL, INR of 3.5, and a potassium of 5.6 mmol/L. Subsequent hepatitis panel was notable for positive HBsAg and anti-HBc antibodies. HBV DNA level was 9.7 million suggestive of reactivation of chronic occult HBV infection. The patient had never received a viral hepatitis screening prior to this hospitalization. His AKI was likely due to a combination of ibrutinib-associated nephrotoxicity and pre-renal injury from volume depletion.
Conclusion
This case reinforces the importance of obtaining baseline Hepatitis B serologies prior to new initiation of all chemotherapeutic agents in patients with hematologic or oncologic diagnoses. It also highlights the less-mentioned effect of ibrutinib on renal function.
HMG-CoA Reductase Inhibitors and the Attenuation of Risk for DIC in Patients with Sepsis*
Nicholas Alana, Internal Medicine Resident; William Ciurylo, DO
Portsmouth Regional Hospital
Background
Disseminated Intravascular Coagulation (DIC) is a life-threatening syndrome characterized by uncontrolled coagulation leading to the consumption of clotting factors and platelets and increasing the risk of life-threatening hemorrhage through mechanisms including dysregulation of anticoagulation pathways and microcirculatory inflammatory processes. Sepsis is a condition that has been shown to increase the risk of the development of DIC. HMG-CoA inhibitors (statins) have demonstrated anti-inflammatory and anti-coagulant properties affecting the pathways proposed to mediate DIC. The aim of this study is to evaluate whether septic patients who take statin medications are at reduced risk for the development of DIC.
Methods
In a retrospective cohort study, 120,397 critically ill patients with sepsis were identified within a three-year period from 2019 to 2021. Patients were stratified by whether they were medicated with low, medium, high-intensity statins, or none. Odds ratios for the development of DIC were calculated.
Results
Overall, 2795 patients would develop DIC. The odds ratio for the development of DIC were .700 (95% CI .617-.794), .615 (95% CI .529-.714), and .901 (95% CI .635-1.277) when comparing high, moderate, and low-intensity statins to no statin therapy.Conclusions
The use of moderate and high-intensity statins was associated with a significantly reduced association between critically ill septic patients and the development of DIC. More research is needed to investigate the potential for this class of medication to be protective against DIC.
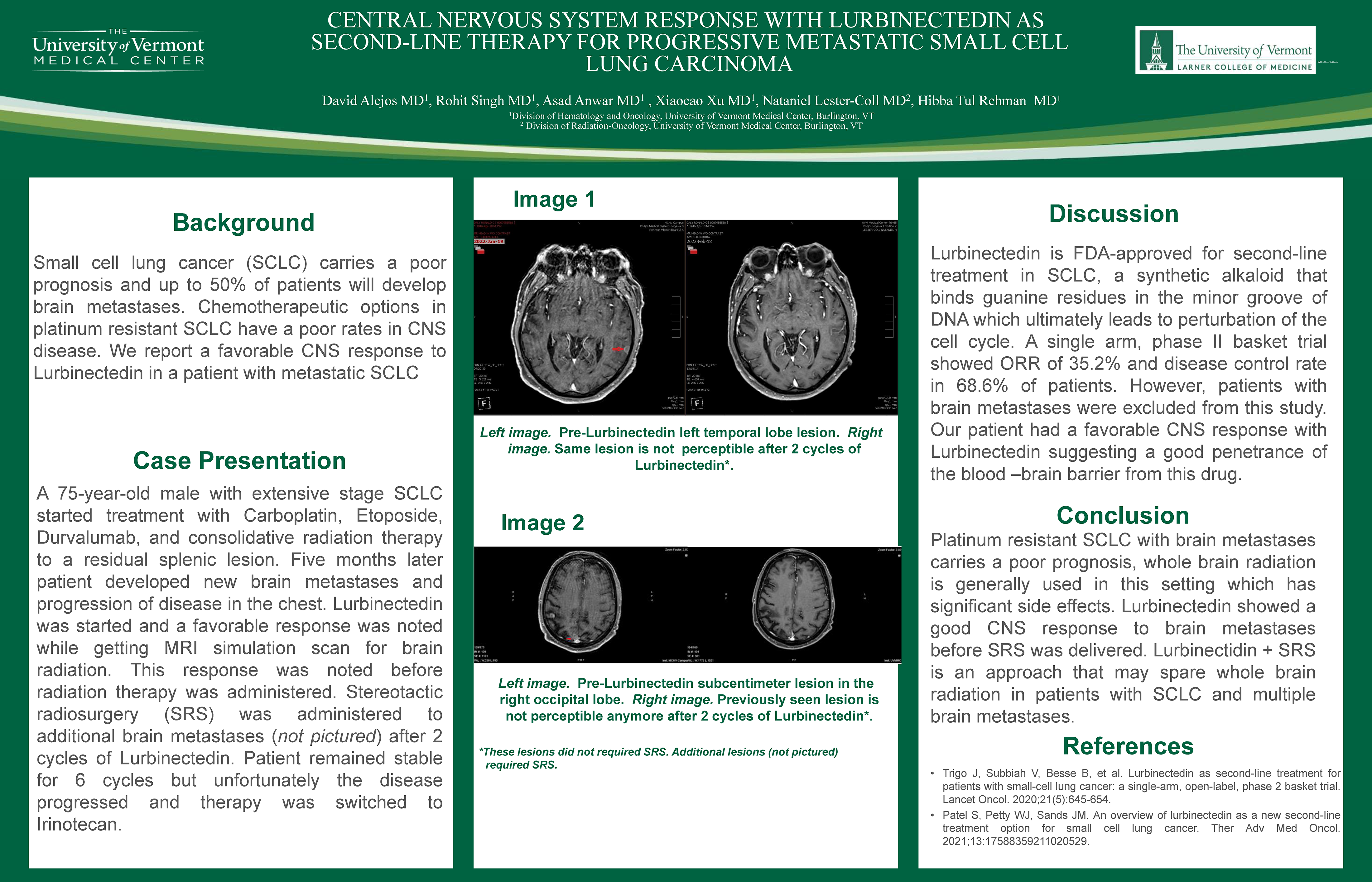
Central Nervous System Response with Lurbinectedin as Second-line Therapy for Progressive Metastatic Small Cell Lung Carcinoma
David Alfonso Alejos Castillo, MD; Rohit Singh; MD, Asad Anwar, MD; Xiaocao Xu; MD, Hibba Rehman
University of Vermont Medical Center
Background
Small cell lung cancer (SCLC) carries a poor prognosis and usually presents with metastatic disease especially to brain. Current chemotherapy has poor efficacy in treating brain metastases suggesting poor penetration of the blood-brain barrier. We report a favorable CNS response to lurbinectedin in a patient with metastatic SCLC.
Methods
A 75 year old male with extensive SCLC started treatment with carboplatin, etoposide, and durvalumab and radiation therapy to a splenic lesion. Five months later patient developed new brain metastases and progression of chest disease. Lurbinectedin was started and a dramatic response was noted while getting CT simulation for brain radiation. Stereotactic radiosurgery to brain metastases was administered after 2 cycles of lurbinectedin. Patient remained stable for six cycles with lurbinectedin but unfortunately the diseased progressed and therapy was switched to irinotecan.
Results
Lurbinectedin is FDA approved for second-line treatment in SCLC, a synthetic alkaloid that binds DNA-protein interactions and RNA transcription. A single arm, phase II basket trial showed an ORR of 35.2% and disease control rate in 68.6% of patients. However, patients with brain metastases were excluded from this study. Our patient had a dramatic and favorable response with lurbinectidin intracranially suggesting a good CNS penetrance from this drug.Conclusions
Platinum resistant SCLC with brain metastasis carries a poor prognosis, whole brain radiation is generally used in this setting which has significant side effects. Lurbinectedin showed a good CNS response in this patient with brain metastases, which to our knowledge is the first report of such response.
Pancreatic mixed acinar neuroendocrine carcinoma: a single institutional genomic characterization report
Manik Amin; Juliana Castellano; Donald Green; Gregory Tsongalis
Dartmouth Hitchcock Medical Center
Background
Pancreatic mixed acinar-neuroendocrine carcinomas are a rare distinctive yet aggressive entity with histologic and immunohistochemical features of pancreatic acinar cell carcinoma and pancreatic neuroendocrine tumor and pose diagnostic and prognostic challenges. Very few cases have been described in the literature. Genomic information about these tumors remains unknown.
Methods
Two patients were identified from the Dartmouth Pathology database with a biopsy proven diagnosis of pancreatic mixed acinar neuroendocrine carcinoma. Genomic characterization (NGS) was done using the Illumina TruSight Tumor 170 (TST170) sequencing assay which detects gene variants across 170 gene targets in nucleic acids extracted from FFPE tissue samples. Sequencing was performed on the NexSeq 500 system which is designed to examine single nucleotide variants, small deletions, small insertions, amplifications, fusions and splice site variants across170 genes.
Results
Two patients identified from the database had tumors in the head of the pancreas. Results are summarized in the table below. Table 1. Histopathologic and NGS characteristics of patients.
Conclusions
Our patient with a higher Ki 67 index had poor prognosis as compared to the patient with the G2 tumor. Mutations such as FGFR1-TACC fusion, BARD1 has been documented in brain tumors and breast cancers respectively. Similarly, DDR2 mutation has been identified as one of the actionable mutations in squamous cell carcinoma of lungs. Significance of these mutations identified in this rare tumor is yet unknown. Our search of Dartmouth pathology database is ongoing to identify more cases in the last 10 years and to complete genomic analysis on these cases.
Renal dedifferentiated chondrosarcoma and PDL1 inhibitor conundrum
Rahul Anil, MD; Haze Xu, MD; Karamatullah Danyal, MD; Alexandra Kalof, MD; Hibba-Tul Rehman, MD
The University of Vermont Medical Center
Background
A rare disease, chondrosarcoma has a poor prognosis and is resistant to both chemotherapy and radiation. Little is known about the susceptibility of specific sarcoma subtypes to immunotherapy.
Methods
We present a case of a 68-year-old female with a left renal mass in the upper pole (7.0 cm) with histological invasion into the renal collecting system, left renal vein, bilateral lung nodule, multiple nodules within bilateral renal parenchyma, and right axillary lymph nodes. Biopsy of lung nodules and left renal mass revealed an S-100 and vimentin positive, PAX-8, GATA3, MDM-2 negative specimen concerning for a dedifferentiated chondrosarcoma. Genomic profiling of the tumor showed it to be TMB 8 and MSI stable which would usually not warrant the use of PDL1 as first line therapy. CT scans revealed initial and continuous decrease or stability in all of the related nodules after cycles 2, 6, and 12.
Results
Not applicable
Conclusions
In conclusion, though chondrosarcomas are generally considered a “non-immunogenic”, our case report corroborates that chondrosarcomas are highly variable in their biology and each case should be considered a separate therapeutic challenge that requires further studies to understand its immune and molecular biology.
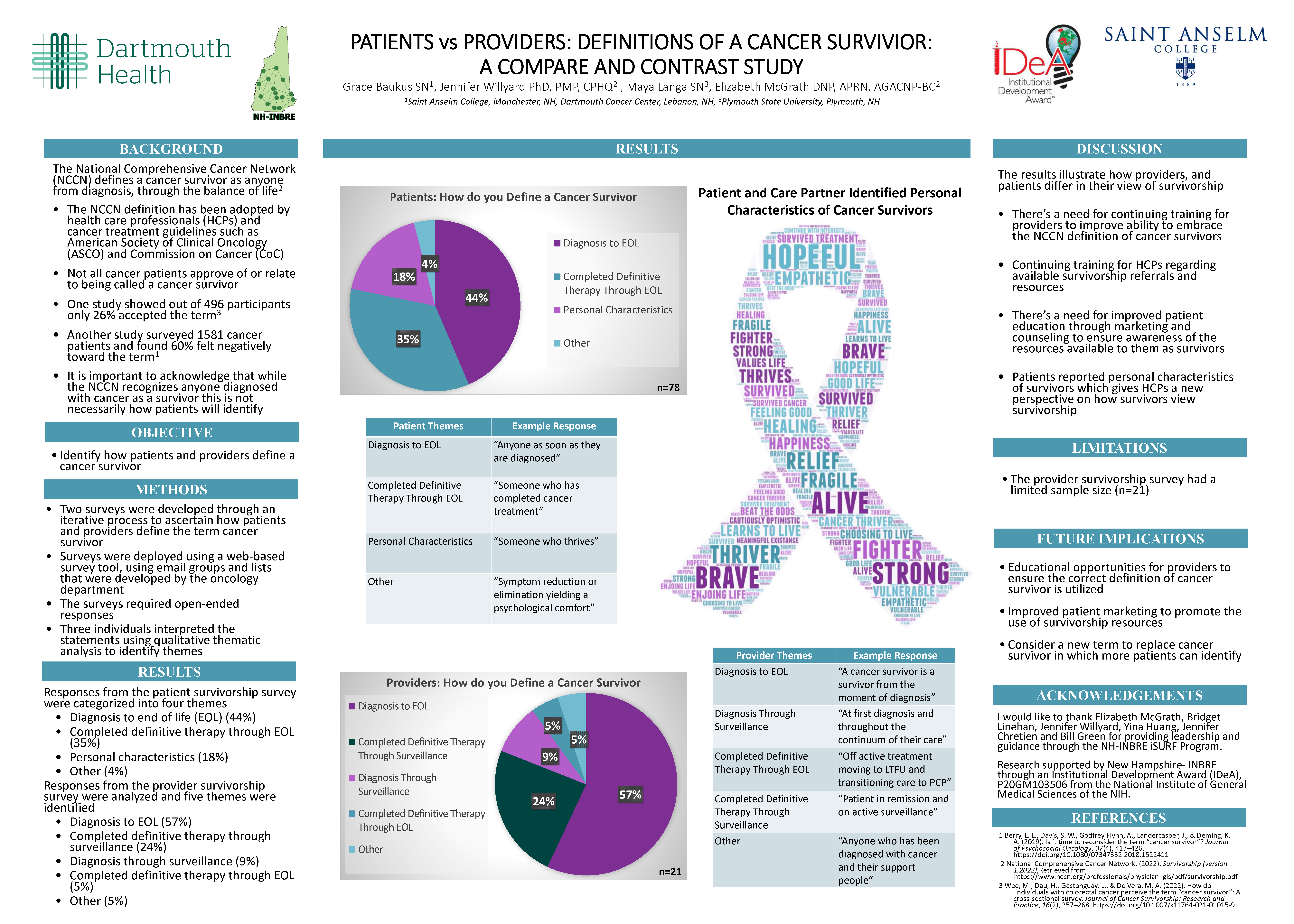
Patients vs Providers: Definitions of a Cancer Survivor: A Compare and Contrast Study
Grace Baukus; Elizabeth McGrath
Saint Anselm College; Dartmouth Cancer Center
Background
According to the National Comprehensive Cancer Network (NCCN) the definition of a cancer survivor is anyone from diagnosis through end of life (EOL). However, not all cancer patients relate to or identify with this designation. The purpose of this study is to compare how patients and health care professionals (HCPs) define a cancer survivor.
Methods
Two surveys were created through an iterative process to ascertain how patients and providers define survivors. Surveys were deployed using a web-based survey tool, using email groups and lists developed by the oncology department. The results were analyzed using qualitative thematic analysis.
Results
The patient survey was analyzed and four themes identified. Two unique themes were identified from the patients’ responses, personal characteristics and other. The provider survey was analyzed and five themes were identified. The providers’ responses coded into three unique themes, completed definitive therapy through surveillance, diagnosis through surveillance and other. The surveys had two shared themes, diagnosis to EOL and completed definitive therapy through EOL. Despite the NCCN standardized definition of a cancer survivor, not all providers or patients utilize this definition.
Conclusions
There are distinct perspectives from both patients and providers around the term cancer survivor. It is important to listen to patients to understand how they identify themselves as survivors. Improving patient marketing and counseling will ensure that patients are aware of the designation of a survivor, as well as the resources available to them. It’s also essential to ensure HCPs have adequate knowledge of survivorship resources for proper referrals and patient education.
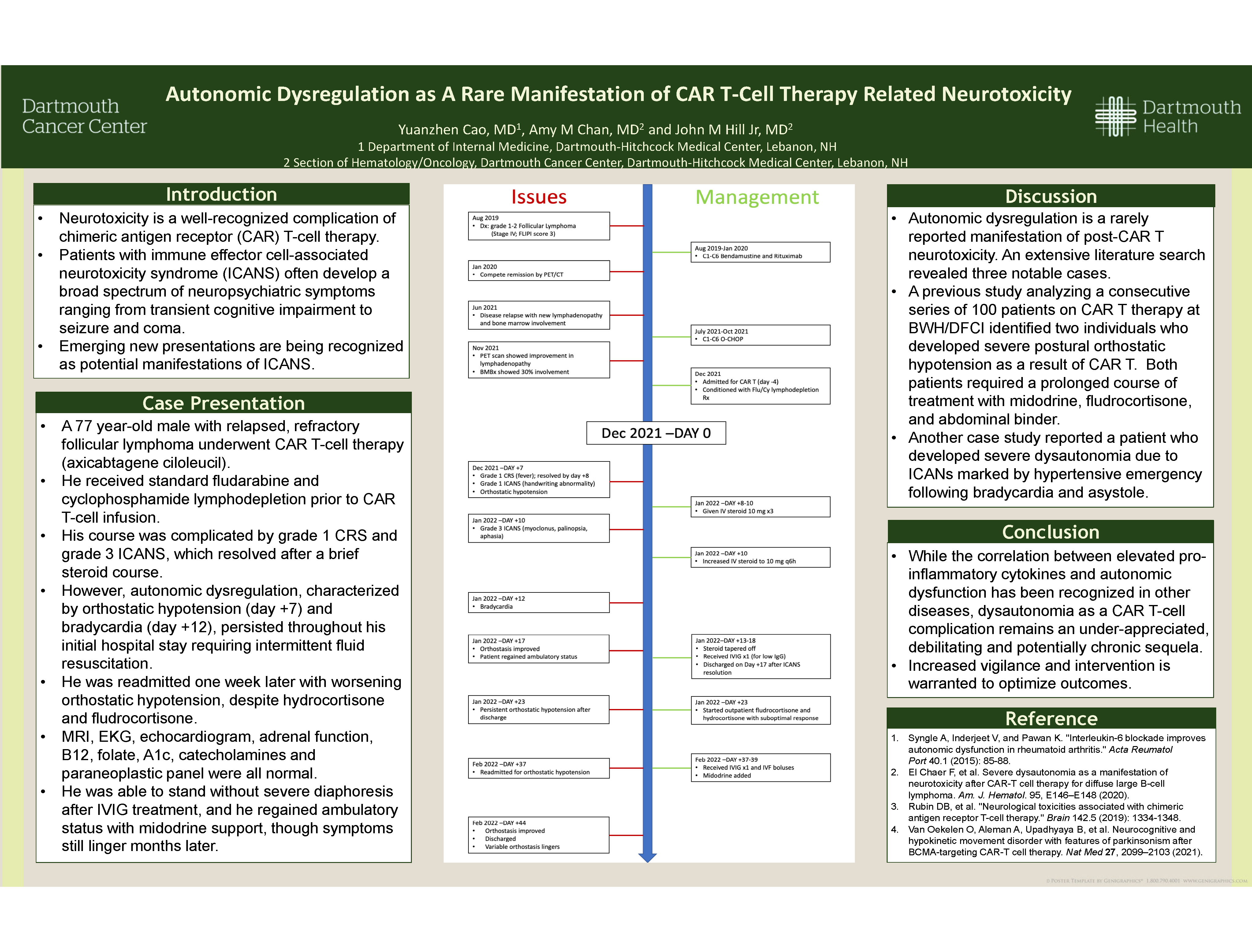
Autonomic Dysregulation as A Rare Manifestation of CAR T-Cell Therapy Related Neurotoxicity
Yuanzhen Cao; Amy M Chan MD; John M Hill Jr MD
Dartmouth-Hitchcock Medical Center
Background
Neurotoxicity is a well-recognized complication of chimeric antigen receptor (CAR) T-cell therapy. Patients with immune effector cell-associated neurotoxicity syndrome (ICANS) often develop a broad spectrum of neuropsychiatric symptoms ranging from transient cognitive impairment to seizure and coma. Emerging new presentations are being recognized as potential manifestations of ICANS.
Methods
We describe a patient who developed autonomic dysregulation due to CAR T-cell therapy.
Results
A 77 year-old male with relapsed, refractory follicular lymphoma underwent CAR T-cell therapy. He received standard fludarabine/cyclophosphamide lymphodepletion prior to CAR T-cell infusion. His course was complicated by grade 1 CRS and grade 3 ICANS (based on transient palinopsia, myoclonus and agraphia), which resolved after a brief steroid course. However, autonomic dysregulation, characterized by orthostatic hypotension (day +7) and bradycardia (day +12), persisted throughout his initial hospital stay requiring intermittent fluid resuscitation. He was readmitted one week later with worsening orthostatic hypotension, despite hydrocortisone and fludrocortisone. MRI, EKG, echocardiogram, adrenal function, B12, folate, A1c, catecholamines and paraneoplastic panel were all normal. He was able to stand without severe diaphoresis after IVIG treatment, and he regained ambulatory status with midodrine support, though symptoms still linger months later.
Conclusions
Autonomic dysregulation is a rarely reported manifestation of post-CAR T neurotoxicity, and our patient’s initial response to IVIG supports this diagnosis. While the correlation between elevated pro-inflammatory cytokines and autonomic dysfunction has been recognized in other diseases, dysautonomia as a CAR T-cell complication remains an under-appreciated, debilitating and potentially chronic sequela. Increased vigilance and intervention is warranted to optimize outcomes.
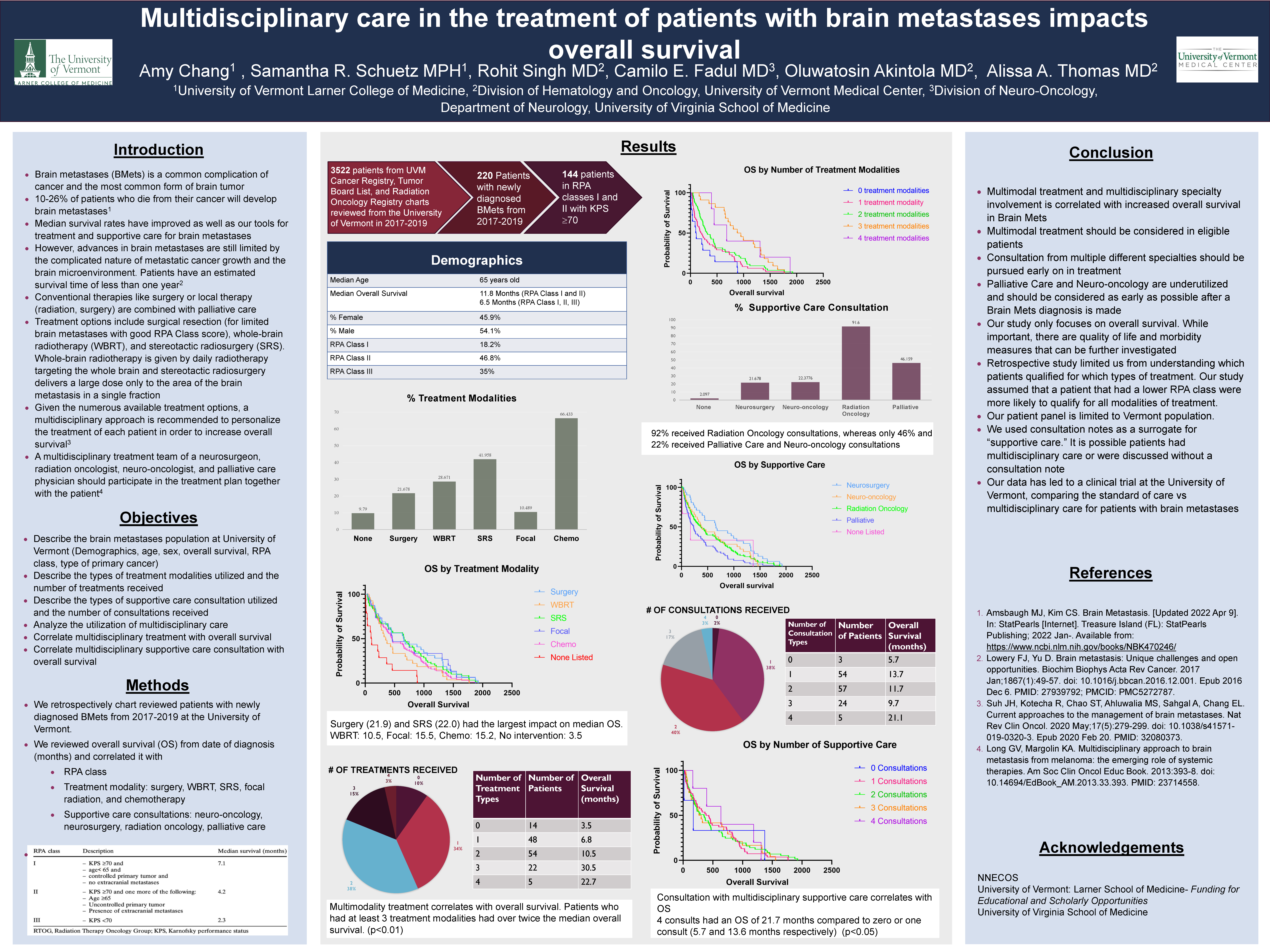
Multidisciplinary care in the treatment of patients with brain metastases impacts
overall survival*
Amy Chang, Samantha Schuetz, Rohit Singh, Camilo E. Fadul, Oluwatosin Akintola,
Alissa A. Thomas
Background
Guideline-based management of brain metastases (BMets) consists of local therapy (surgery, radiation) for symptomatic BMets, and systemic therapy and/or local therapy for asymptomatic BMets in patients with adequate performance status. We performed a retrospective quality review of multidisciplinary treatment utilization.
Methods
We retrospectively reviewed patients with newly diagnosed BMets from2017-2019 at University of Vermont and reviewed overall survival (OS) from date of diagnosis (months) by RPA class, treatment modality (surgery, stereotactic radiosurgery, whole brain radiation, and systemic therapy), and multidisciplinary care consultations (neuro-oncology, neurosurgery, radiation oncology, palliative care).
Results
We identified 220 patients with BMets with a follow up of 6 months (or until death) and included 144 patients in RPA classes I and II with KPS 70 in our analysis. The median OS was 11.8 months. Patients who received multi-modal treatment had significantly improved OS (4 modalities= 22.7, 3 modalities= 30.5, 2 modalities=10.5, 1 modality=6.8, 0 modalities=3.5, p<0.01). Surgery and stereotactic radiosurgery had the largest impact on OS (21.9 and 22.0). Consultation with multiple specialty services correlated with increased survival, with patients who received 4 consults (neurooncology, neurosurgery, radiation oncology, palliative care) demonstrating an OS of 21.7 months, while patients who received zero or one consult had an OS of 5.7 and 13.6 months respectively (p<0.05). 92% received Radiation Oncology consultations, whereas only 46% and 22% received Palliative care and Neuro-oncology consultations, respectively.
Conclusions
Multimodal treatment and multidisciplinary specialty involvement is correlated with increased OS in BMets for patients with an RPA classes I or II.
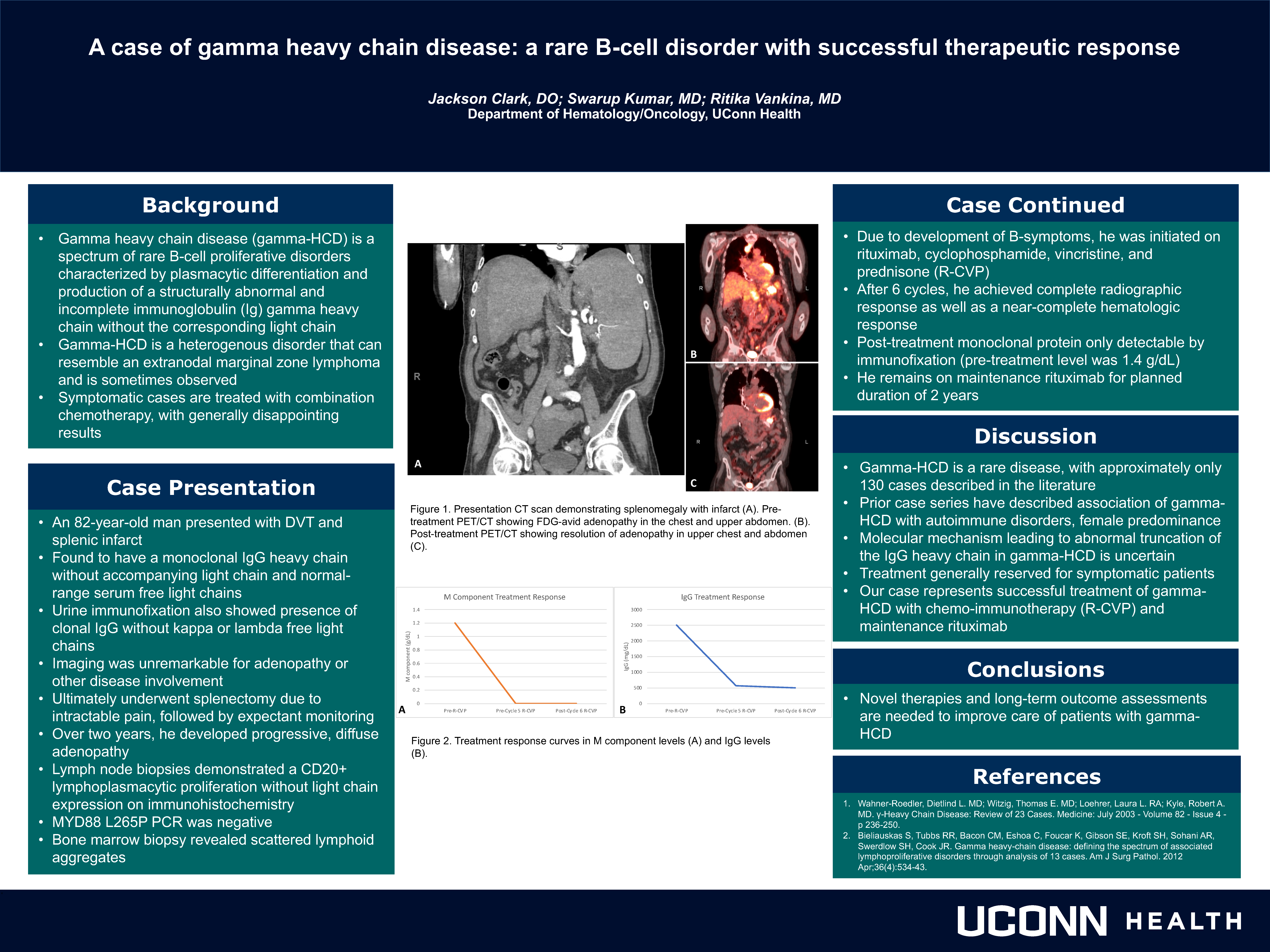
A case of gamma heavy chain disease: a rare B-cell disorder with successful therapeutic response
Jackson Clark; Ritika Vankina, MD; Swarup Kumar, MD
UCONN Department of Hematology and Medical Oncology
Background
Gamma heavy chain disease (gamma-HCD) is a rare B-cell proliferative disorder characterized by production of a structurally abnormal and incomplete immunoglobulin (Ig) gamma heavy chain without the corresponding light chain. Gamma-HCD is a heterogenous disorder that can resemble an extranodal MALT lymphoma and is sometimes observed. Symptomatic cases are treated with combination chemotherapy, with generally disappointing results.
Methods
An 82-year-old man presented with DVT and splenic infarct and was found to have a monoclonal IgG heavy chain without accompanying light chain and normal-range serum free light chains. Urine immunofixation also showed the presence of clonal IgG without kappa or lambda free light chains. Comprehensive imaging was unremarkable for adenopathy, and he ultimately underwent splenectomy due to intractable pain, followed by expectant monitoring. Over two years, he developed progressive, diffuse adenopathy. Lymph node biopsies demonstrated a CD20+ lympho-plasmocytic proliferation without light chain expression on immunohistochemistry. MYD88 L265P PCR was unremarkable. Bone marrow biopsy revealed scattered lymphoid aggregates. As the patient had B-symptoms, he was initiated on rituximab, cyclophosphamide, vincristine, and prednisone. After 6 cycles, he achieved complete radiographic response as well as a near-complete hematologic response, and he remains on maintenance rituximab.
Results
This is a case of gamma-HCD, of which only approximately 130 cases have been described. Our case represents successful treatment of gamma-HCD with chemo-immunotherapy (R-CVP) and maintenance rituximab.
Conclusions
Novel therapies and long-term outcome assessments are needed to improve care of patients with gamma-HCD.
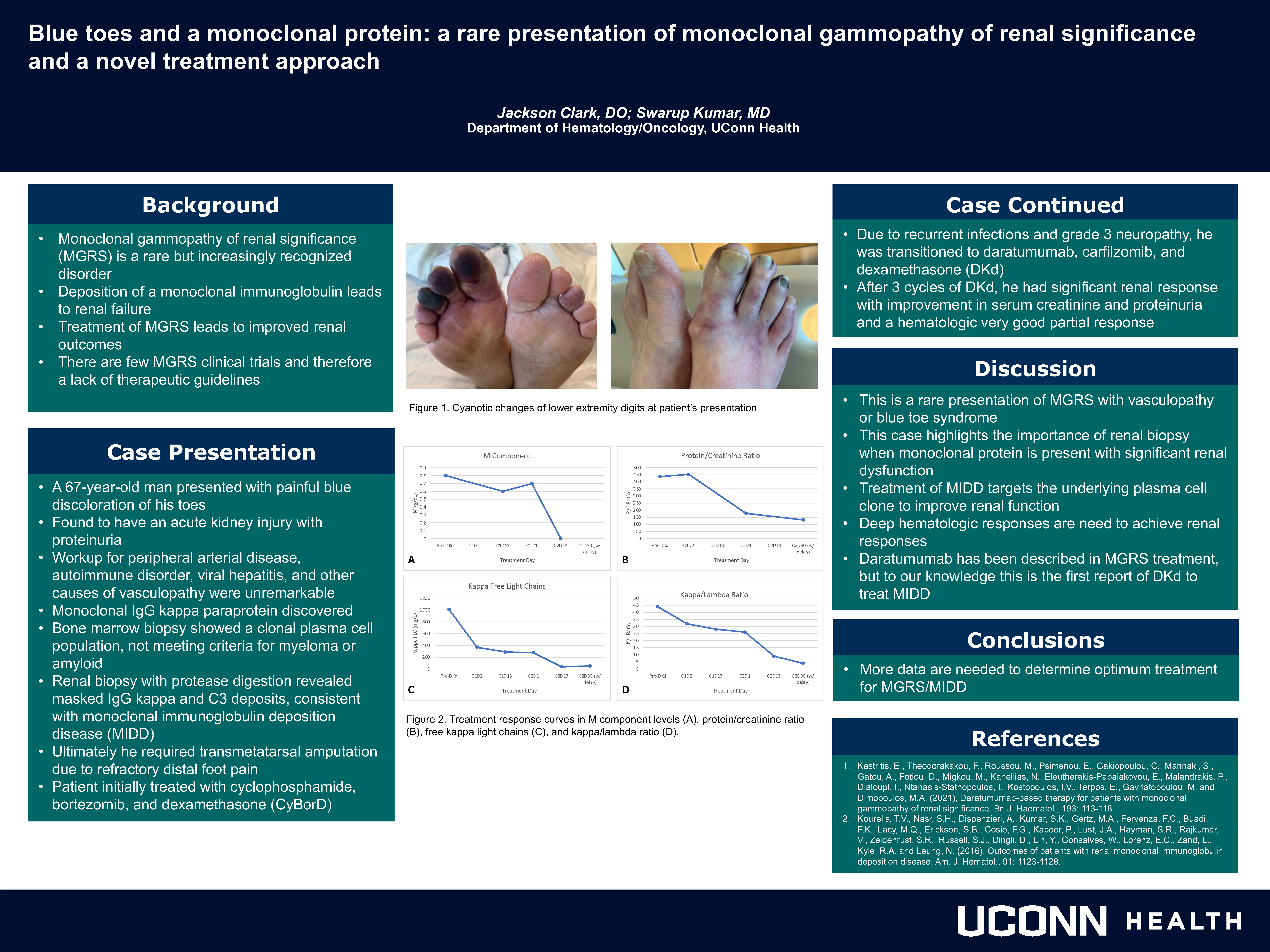
Blue toes and monoclonal protein: a rare presentation of monoclonal gammopathy of renal significance and a novel treatment approach
Jackson Clark; Swarup Kumar, MD
UCONN Department of Hematology and Medical Oncology
Background
Monoclonal gammopathy of renal significance (MGRS) is a rare but increasingly recognized disorder where deposition of a monoclonal immunoglobulin leads to renal failure. While treatment of MGRS leads to improved renal outcomes, there are few MGRS clinical trials and therefore a lack of therapeutic guidelines.
Methods
A 67-year-old man presented with painful blue discoloration of his toes and was found to have kidney injury with proteinuria. Extensive vasculopathy workup was unremarkable but did reveal a serum IgG kappa paraprotein. Bone marrow biopsy showed a clonal plasma cell population, not meeting criteria for myeloma or amyloid. Renal biopsy with protease digestion revealed masked IgG kappa and C3 deposits, consistent with monoclonal immunoglobulin deposition disease (MIDD). He started treatment with cyclophosphamide, bortezomib, and dexamethasone (CyBorD). Due to recurrent infections and grade 3 neuropathy, he was transitioned to daratumumab, carfilzomib, and dexamethasone (DKd). After 3 cycles of DKd, he had significant renal response with improvement in serum creatinine and proteinuria and a hematologic very good partial response.
Results
This is a rare presentation of MGRS with pseudogangrene and highlights the importance of renal biopsy. Daratumumab has been described in MGRS treatment, but to our knowledge this is the first report of DKd to treat MIDD.
Conclusions
Patients with a monoclonal protein and significant renal dysfunction should undergo renal biopsy to rule out MGRS. Treatment of MIDD targets the underlying plasma cell clone to improve renal function.
Feasibility and Results of a Multidisciplinary Clinic for GI Malignancy in a Community Hospital Setting
Karin Cole, MD; Zachary Cost
Northern Light Mercy Surgery; Tufts University School of Medicine
Background
A multidisciplinary approach has been established as the standard of care for a wide variety of malignant diagnoses. While multidisciplinary discussion at regularly scheduled tumor board meetings is a common way to provide such care, true multidisciplinary clinics are less common, particularly in the community hospital setting. We established a multidisciplinary clinic (MDC) for GI malignancy at Mercy in 2019, and here report results from the first 44 patients.
Methods
A database of basic patient information (including clinic date, diagnosis, providers seen, and treatments completed) was maintained for all patients presenting to MDC. Treatment dates were abstracted from the medical record.
Results
44 patients were seen between September 2019 and June 2022. The most common diagnoses were colon (20) and rectal (12) cancer. In addition to the medical and surgical oncologist, 27 (61%) patients saw the oncology social worker, 22 (50%) saw the dietician, and 9 (20%) saw the genetic testing coordinator. 30 patients underwent surgical intervention, 20 had systemic therapy, and 8 underwent radiation therapy. Average time to initial surgical therapy was 21 days from date of MDC; for initial systemic therapy, average time was 34 days.
Conclusions
True multidisciplinary care can be provided in a community hospital clinic setting, increasing patient convenience, and providing earlier opportunities for collaboration and discussion among specialties.
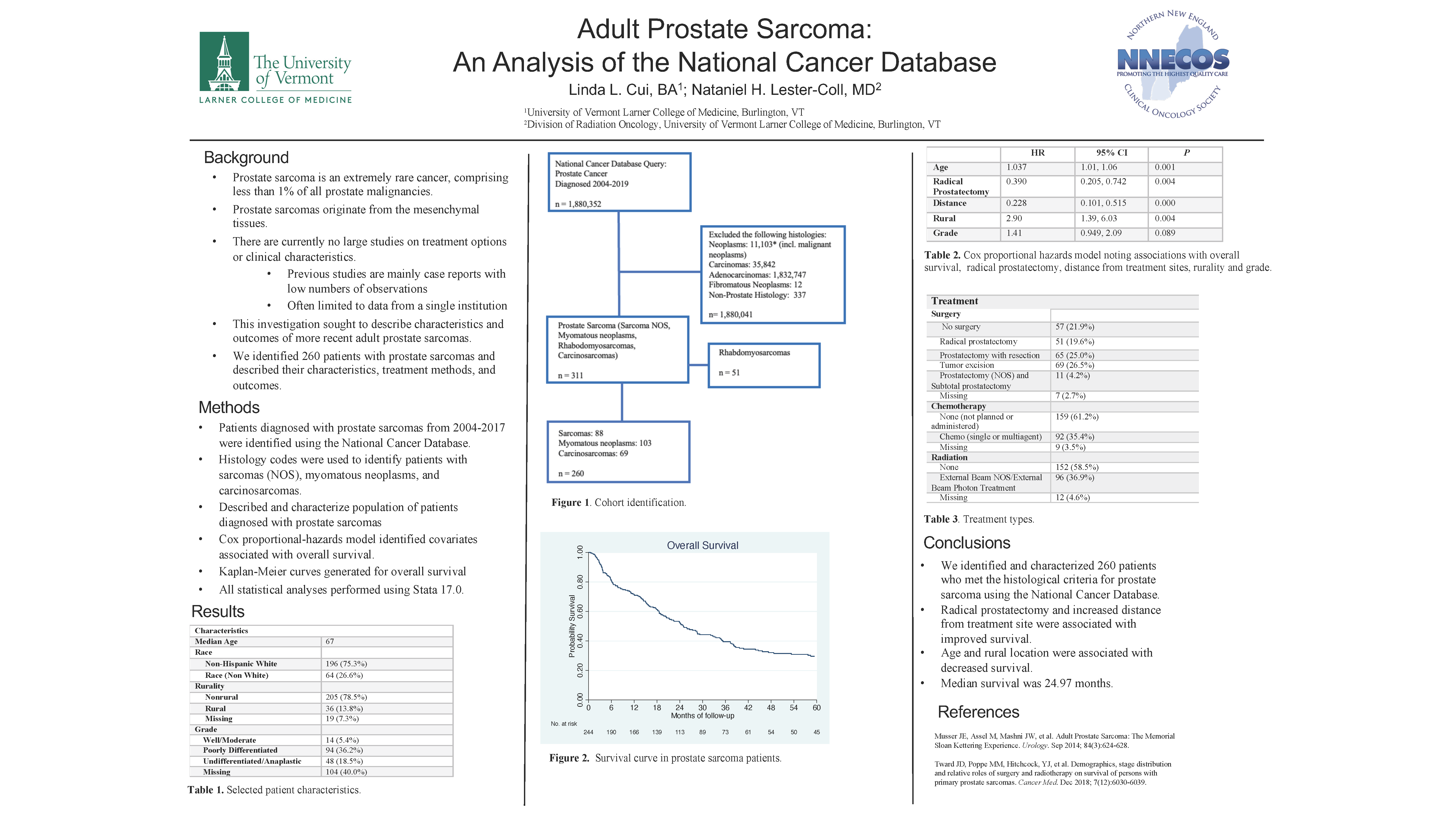
Adult Prostate Sarcoma: An Analysis of the National Cancer Database
Linda Cui, B.A.; Nataniel Lester-Coll
University of Vermont
Background
Adult prostate sarcoma is an extremely rare cancer, comprising fewer than 1% of all prostate malignancies. Due to their rarity, there is a paucity of data describing treatment patterns and clinical characteristics that may affect patient outcomes. We evaluated the demographics, treatment selections and outcomes of patients with prostate sarcomas using a nationally representative sample.
Methods
This is a retrospective study using the National Cancer Database of patients with prostate sarcomas diagnosed from 2004-2019. We used descriptive statistics to describe these patients. Logistic regression was used to describe factors associated with surgical treatment. Kaplan-Meier analysis was used to describe overall survival (OS). Cox proportional modeling was used to identify factors associated with survival.
Results
Using histological criteria, we identified 260 patients with prostate sarcomas. The median age at diagnosis was 67 years. Median survival was 24.97 months (95% CI 20.3-32.62). 36.67% of patients were treated with chemotherapy. 77.47% were treated with surgery. 38.7% were treated with radiation. On multivariable analysis, factors associated with improved survival were increased distance from treatment site (HR = 0.228, p = 0.000) and radical prostatectomy (HR = 0.390, p=0.004). Age (HR = 1.037, p=0.001) and rural location (HR = 2.90, p = 0.004) are associated with decreased survival. Undifferentiated and anaplastic grade was marginally associated with poor survival (HR = 1.41 , p=0.089).
Conclusions
Adult prostate sarcomas are a rare cancer with poor prognosis. Surgical treatment options may be associated with a better overall survival.
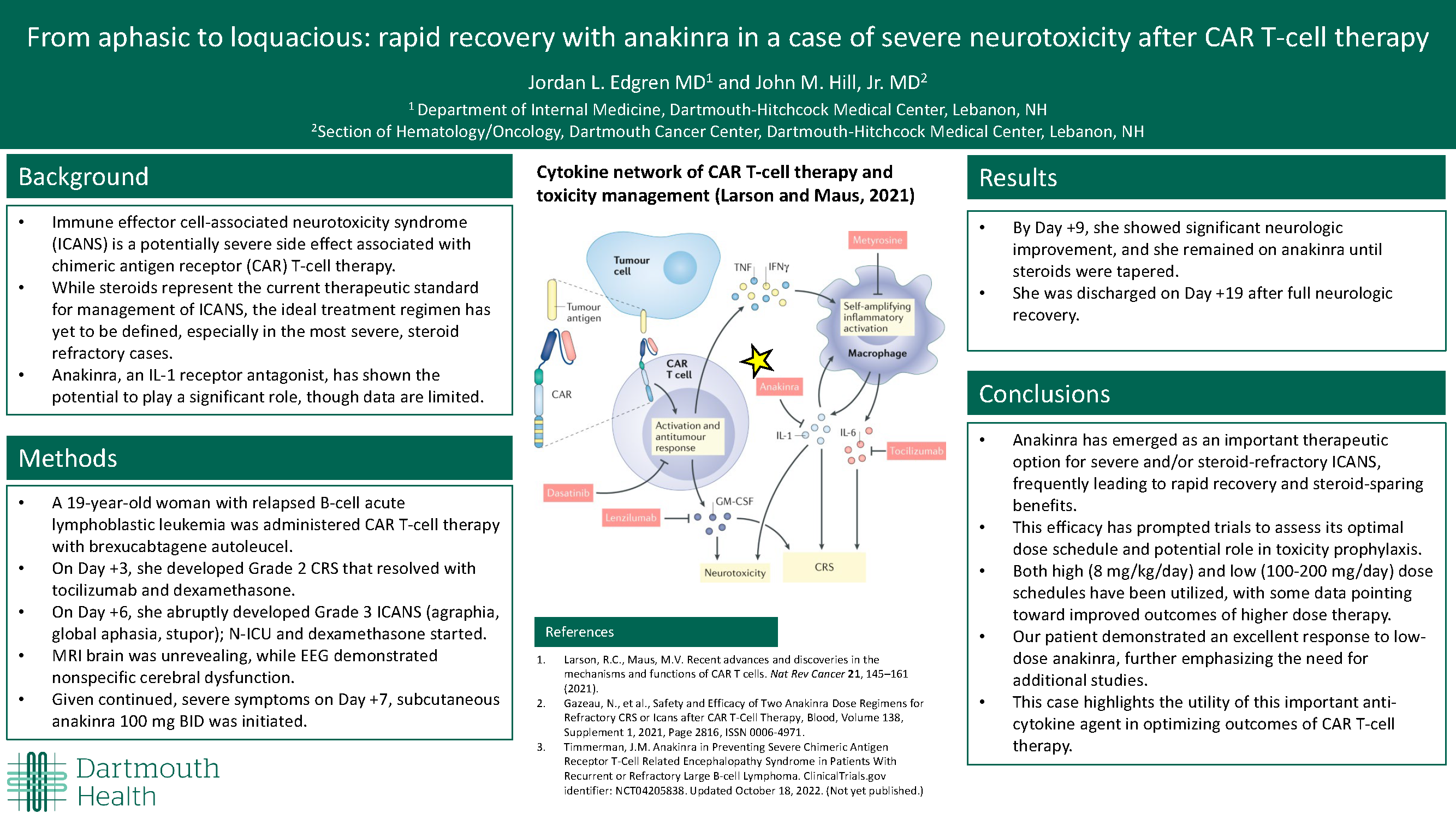
From aphasic to loquacious: rapid recovery with anakinra in a case of severe neurotoxicity after CAR T-cell therapy
Jordan Edgren, MD; John M Hill Jr MD
Dartmouth Hitchcock Medical Center
Background
Immune effector cell-associated neurotoxicity syndrome (ICANS) is a potentially severe side effect associated with chimeric antigen receptor (CAR) T-cell therapy. While steroids represent the current therapeutic standard for management of ICANS, the ideal treatment regimen has yet to be defined, especially in the most severe, steroid refractory cases. However, anakinra, an IL-1 receptor antagonist, has shown the potential to play a significant role.
Methods
A 19 year-old woman with relapsed B-cell acute lymphoblastic leukemia presented for CAR T-cell therapy. On Day +3 she developed Grade 2 CRS that resolved with tocilizumab and dexamethasone. On Day +6 she abruptly developed Grade 3 ICANS (agraphia, global aphasia, stupor) warranting dexamethasone. MRI brain was unrevealing while EEG demonstrated nonspecific cerebral dysfunction. Given continued severe symptoms on Day +7, subcutaneous anakinra 100mg BID was initiated.
Results
By Day +9 she showed significant neurologic improvement and she remained on anakinra until steroids were tapered. She was discharged on Day +19 after full neurologic recovery.
Conclusions
Anakinra has emerged as an important therapeutic option for severe and/or steroid-refractory ICANS, frequently leading to rapid recovery and steroid-sparing benefits. This efficacy has prompted trials to assess its optimal dose schedule and potential role in toxicity prophylaxis. Both high (8mg/kg/day) and low (100-200mg/day) dose schedules have been utilized, with some data pointing toward improved outcomes of higher dose therapy. Our patient demonstrated an excellent response to low-dose anakinra, further emphasizing the need for additional studies. This case highlights the utility of this anti-cytokine agent in optimizing outcomes of CAR T-cell therapy.
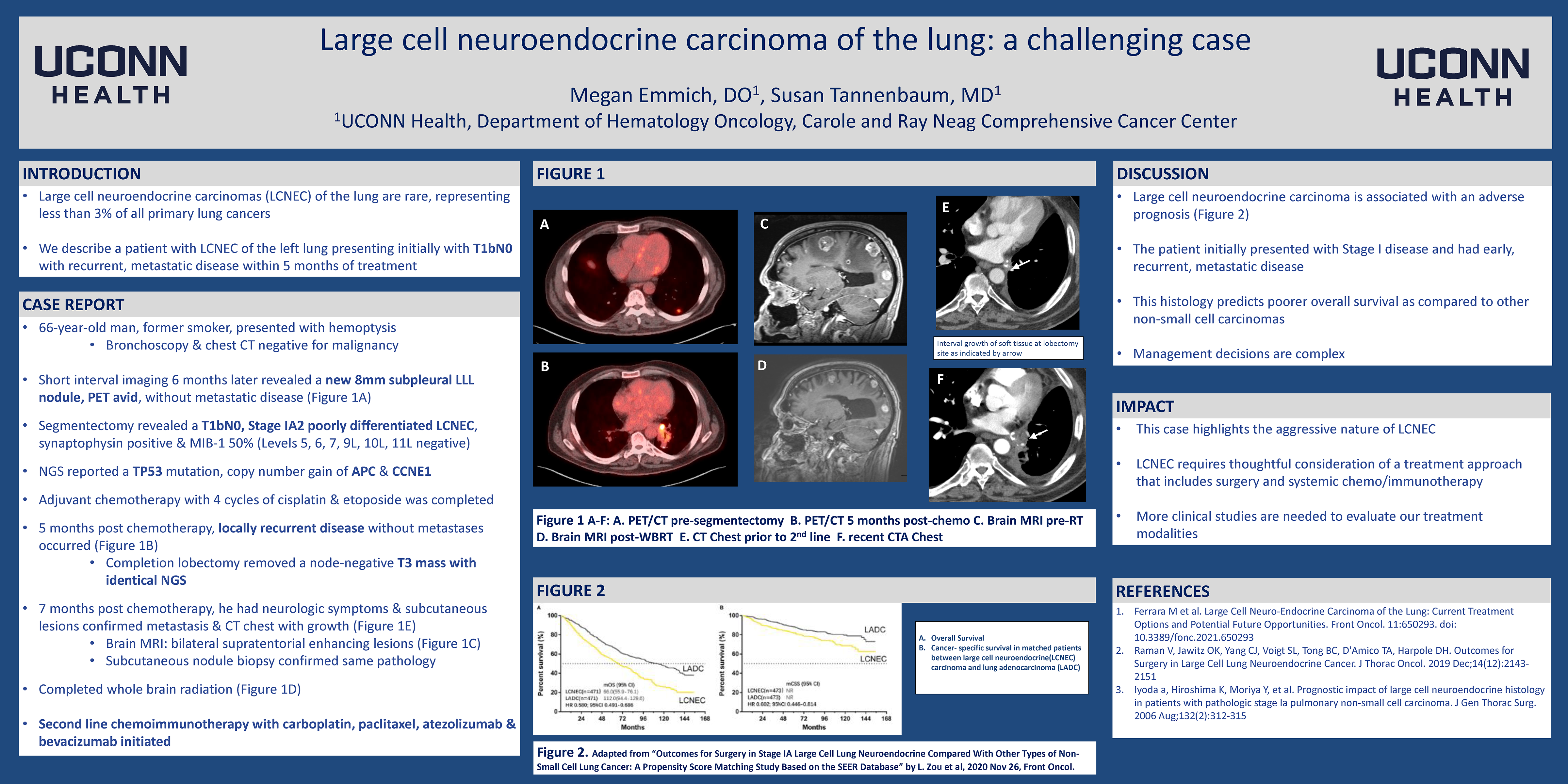
Large cell neuroendocrine carcinoma of the lung: a challenging case
Megan Emmich, DO; Megan Emmich; DO & Susan Tannenbaum, MD
UCONN Health
Background
Large cell neuroendocrine carcinomas (LCNEC) of the lung are rare, representing less than 3% of all primary lung cancers. This histology predicts poorer overall survival as compared to other non-small cell carcinomas. Management decisions are therefore complex and not guideline driven.
Methods
We describe a patient with LCNEC of the left lung with recurrent, metastatic disease.
Results
A 66-year-old man, former smoker, presented with hemoptysis with bronchoscopy and chest CT negative for malignancy. Short interval imaging 6 months later revealed a new 8mm subpleural LLL nodule, PET avid, without metastatic disease. Segmentectomy revealed a T1bN0, Stage IA2 poorly differentiated LCNEC, synaptophysin positive and MIB-1 50%. Next generation sequencing (NGS) reported a TP53 mutation and copy number gain of APC and CCNE1. Despite the early stage, adjuvant chemotherapy with 4 cycles of cisplatin and etoposide was completed. Five months post chemotherapy, locally recurrent disease without metastases occurred. A completion lobectomy removed a node-negative T3 mass with identical NGS. Seven months post chemotherapy, he had neurologic symptoms and subcutaneous lesions which confirmed metastasis. Brain MRI revealed bilateral supratentorial and infratentorial enhancing lesions. He completed whole brain radiation and has been started on second line chemoimmunotherapy with carboplatin, paclitaxel, atezolizumab and bevacizumab.
Conclusions
Large cell neuroendocrine carcinoma is associated with an adverse prognosis. The patient initially presented with Stage I disease and had early, recurrent, metastatic disease. This case highlights the aggressive nature of LCNEC requiring a non-conventional approach.
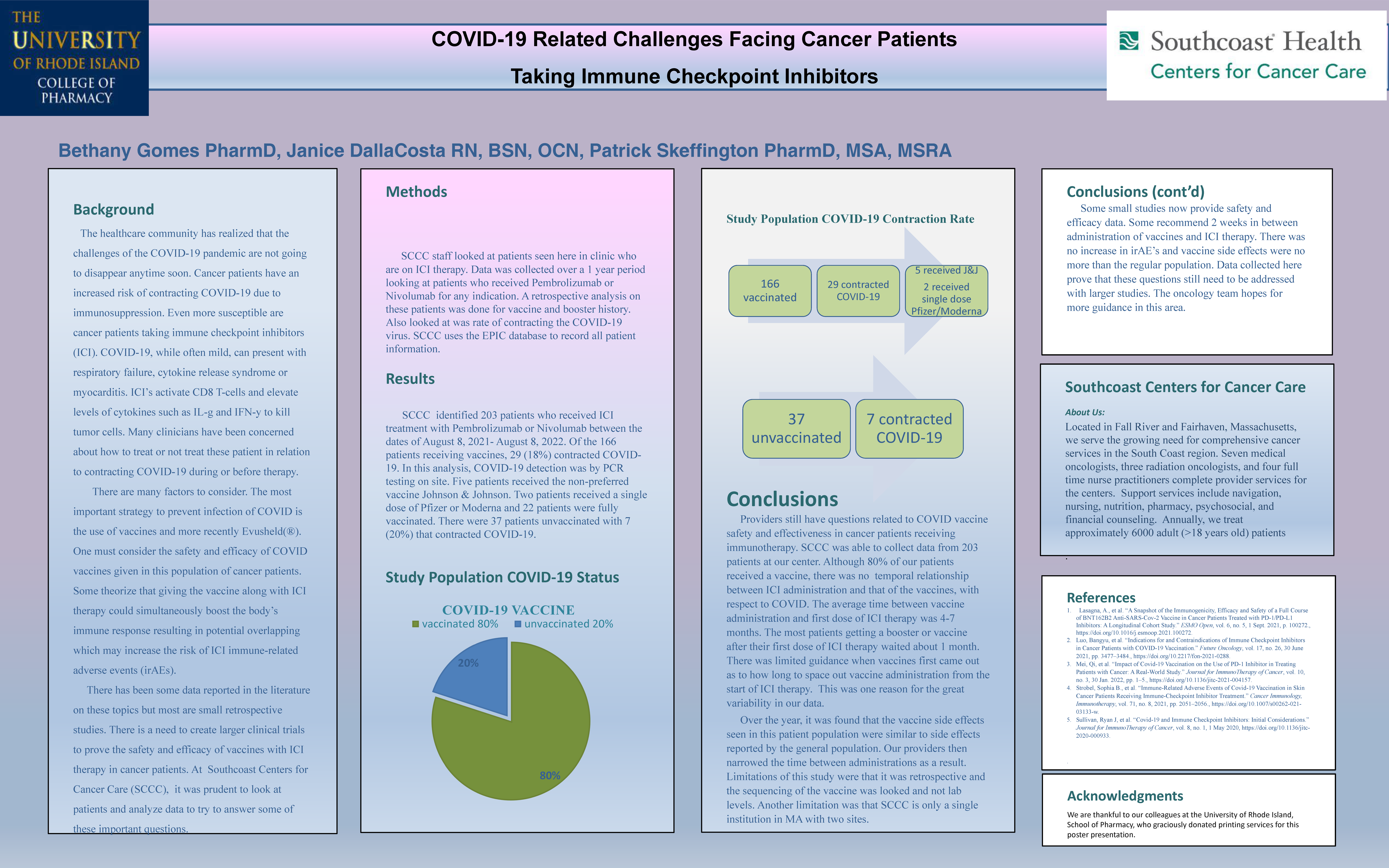
COVID-19 Related Challenges Facing Cancer Patients Taking Immune Checkpoint Inhibitors at SCCC
Bethany Gomes, Pharm. D.; Patrick Skeffington; Janice DallaCosta
Southcoast Cancer Centers
Background
Cancer patients have an increased risk of contracting COVID-19 due to immunosuppression especially those taking immune checkpoint inhibitors (ICI). We must consider the safety and efficacy of COVID vaccines given in this population of cancer patients.
Methods
SCCC collected data over a 1 year period looking at patients who received Pembrolizumab and/or Nivolumab for any indication. We then did a retrospective analysis on these patients for vaccine and booster history. We also looked at rate of contracting the COVID-19 virus.
Results
At SCCC we identified 203 patients who received ICI treatment with Pembrolizumab or Nivolumab between the dates of August 8, 2021- August 8, 2022. Out of the 166 patients receiving vaccines, 29 (18%) contracted COVID-19. Five patients received the non- preferred vaccine Johnson & Johnson. Two patients received a single dose of Pfizer or Moderna and 22 patients were fully vaccinated. There were 37 patients unvaccinated with 7 (20%) that contracted COVID-19.
Conclusions
SCCC collected data on 203 patients at our center. We did not find a temporal relationship between ICI administration, and that of the vaccines, with respect to COVID. Limitations of this study were that it was retrospective and we looked at sequencing of the vaccine and not lab levels. Another limitation was that SCCC is only a single institution in MA with two sites. Our data proves that these questions still need to be addressed with larger studies.
A Case of Severe Vitamin B12 Deficiency
Jasmin Hundal; Dr. Enrique Ballesteros; Dr. Victoria Forbes
University of Connecticut
Background
Vitamin B12 is an essential co-factor in metabolic pathways of hematological and neurological function. Acute presentation of severe vitamin B12 deficiencies can display features of acute myeloid leukemia and thrombotic thrombocytopenic purpura (TTP).
Methods
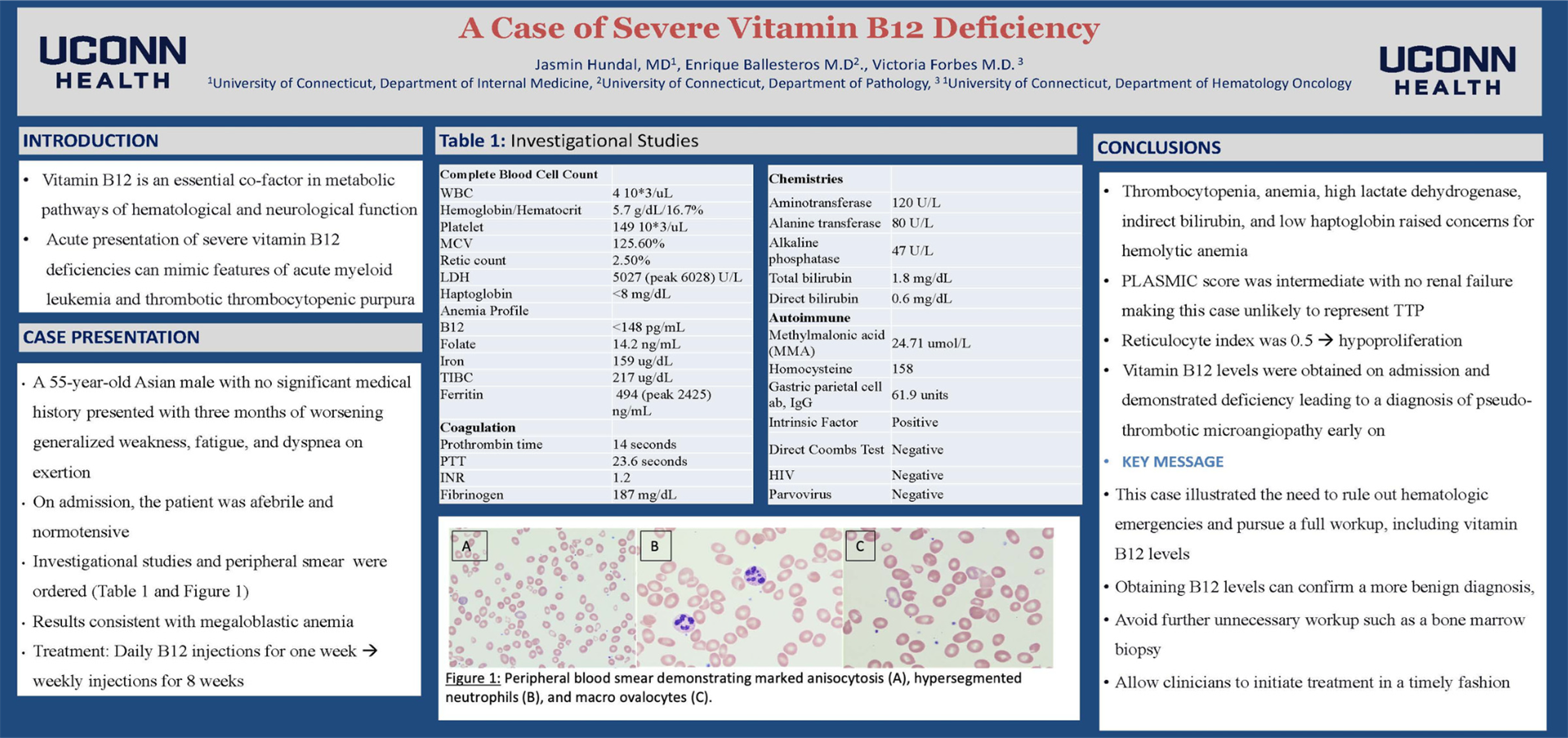
Our patient is a 55-year-old Asian male with no significant medical history presenting with three months of worsening weakness, fatigue, and dyspnea on exertion. He reported a globus sensation and 20 pounds of intentional weight loss. He avoids eating red meat. On admission, his labs were notable for Hb 5.7, MCV 125.6, LDH 5027, haptoglobin <8, B12 <148, reticulocytes 2.5%, negative DAT (Table 1). His smear showed evidence of hemolysis and megaloblastic anemia (Figure 1).
Results
Severe B12 deficiency can cause a pseudothrombotic angiopathy (pseduo-TMA). Hemolysis and signs of MAHA raised concern for TTP in our patient. An intermediate PLASMIC score in the absence of renal failure, thrombocytopenia, and altered mentation made TTP less likely. He also had a hypoproliferative macrocytic anemia with a low B12. He began B12 injections. His gastric parietal IgG antibodies returned as elevated and intrinsic factor was positive leading to a diagnosis of Pernicious Anemia.
Conclusions
This case illustrates the importance of ruling out hematologic emergencies and thoroughly evaluating cytopenias. Obtaining B12 levels can confirm a more treatable, benign diagnosis, avoid unnecessary workup such as a bone marrow biopsy, and allow clinicians to initiate prompt treatment.
Sociodemographic and Occupational Factors associated with receipt of Work Accommodations among employed Breast Cancer Survivors
Jasmin Hundal, M.S., M.D.; Dr. Helen Swede; Dr. Alicia Dugan
University of Connecticut Health Center
Background
Many cancer survivors face occupational challenges when staying in or returning to work after treatment, yet little is known about sociodemographic and work-related factors associated with the receipt of work accommodations.
Methods
We analyzed data from the PinkWorks study, a cross-sectional online survey. Participants were: ≥18+ years, speak English, ended treatment within 36 months, and working ≥20 hours per week at both the time of diagnosis and currently. We performed descriptive analyses to identify possible univariate associations between occupational factors and granting of work accommodations. An exploratory multiple Logistic Regression was also performed.
Results
Among participants, 43.8% received work accommodations from employers, and 11% reported that their physicians had a direct conversation with their employer. Participants who were native English speakers were more likely to receive work accommodations compared to those where English is a second language (44.8%, 22.2%). Workers who held executive positions were more likely to receive accommodations compared to those in either supervisory or non-supervisory roles (60.0%, 43.1%, 42.9%). We observed a higher prevalence of work accommodations among participants who reported that a discussion between the physician and employer occurred compared to those who did not report such a conversation (58%, 41.9%).
Conclusions
Less than half of employed breast cancer survivors were provided work accommodations. Suggestive disparities in the receipt of accommodations were observed among those who were not native English speakers or in non-executive positions. The occurrence of a conversation between the participant’s physician and employer was linked to a higher prevalence of accommodations.
Assessment of Cancer Survivor Needs, Distress Levels and Patient Satisfaction Pre and Post Interprofessional Cancer Survivorship Clinic Visit: A Program Evaluation
Sarah Loschiavo, DNP, APRN, FNP-C, ACHPN; Lisa M. Holle, PharmD, BCOP, FHOPA, FISOPP
UConn Health; UConn School of Pharmacy
Background
Addressing survivorship needs is a priority issue for cancer programs. In 2020, a new survivorship model of care was developed and implemented at UConn Health. Program evaluation is essential to increase high-quality, effective programs. In this program evaluation, the goal was to assess survivorship concerns, patient sense of wellbeing, patient satisfaction and NCCN distress thermometer levels pre- and post-visit.
Methods
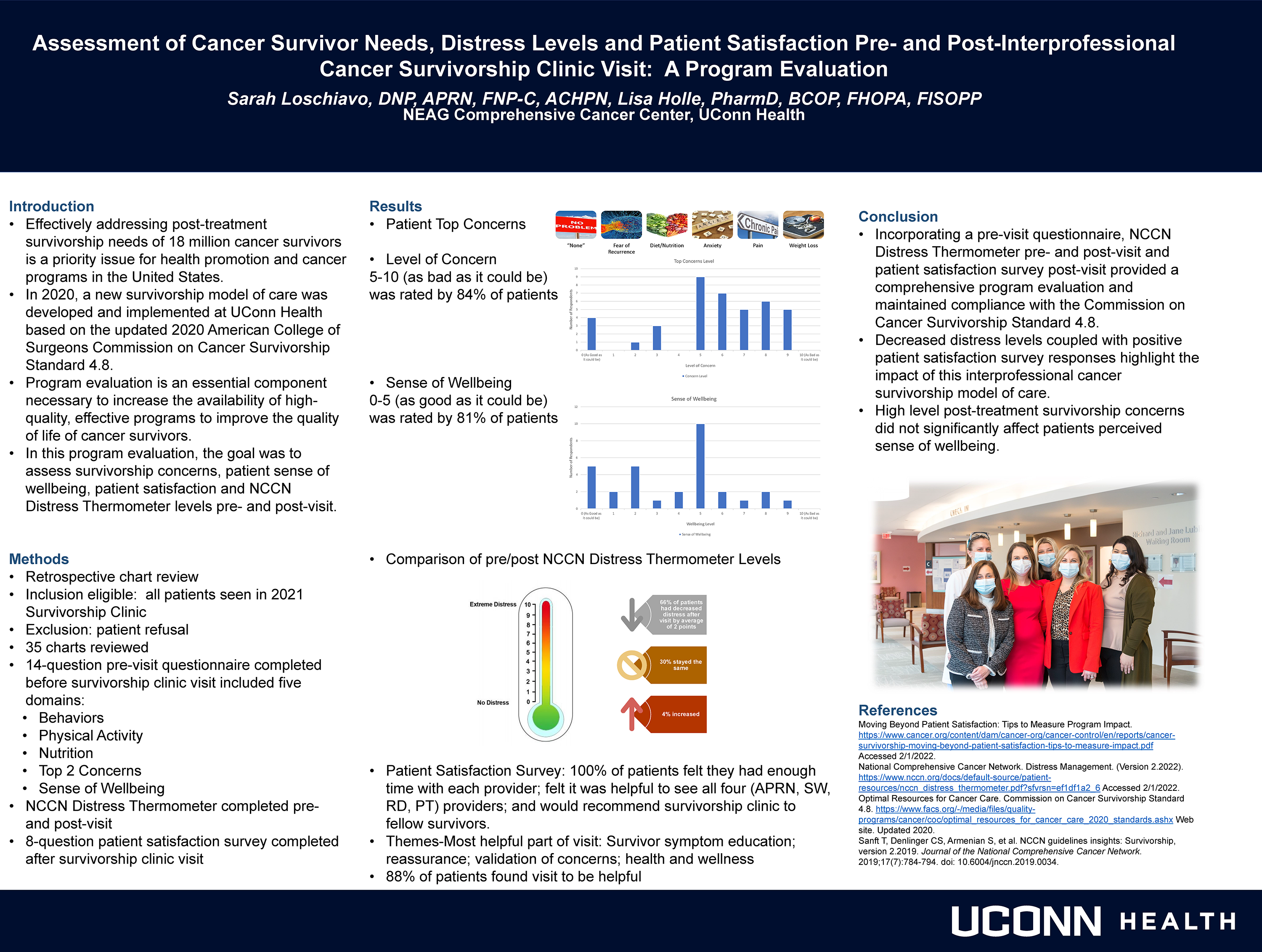
Retrospective chart review. All patients seen in Survivorship Clinic 2021 inclusion eligible. Exclusion were patient refusal. Thirty-five charts reviewed. 14-question pre-visit questionnaire completed before clinic visit included five domains: behaviors; physical activity; nutrition; concerns; wellbeing. NCCN Distress Thermometer completed pre- and post-visit. 8-question patient satisfaction survey completed after clinic visit.
Results
Patient top concerns: “none”, fear of recurrence, diet/nutrition, anxiety, pain, weight loss. Level of Concern 5-10 rated by 84% of patients; 81% rated Sense of Wellbeing 0-5. Comparison of pre/post NCCN Distress Thermometer Levels, 65% had decreased distress by average of 2 points; 30% stayed the same and 4% increased. In the patient satisfaction survey, 100% felt they had enough time with each provider; felt it was helpful to see all four (APRN, SW, RD, PT) providers; and would recommend survivorship clinic to fellow survivors.
Conclusions
Incorporating a pre-visit questionnaire, NCCN distress thermometer pre- and post-visit and patient satisfaction survey following visit provided a comprehensive program evaluation and maintained compliance with the Commission on Cancer survivorship standard. Decreased distress levels coupled with positive patient satisfaction survey responses highlight the impact of this interprofessional cancer survivorship model of care.
Shift in Survivorship Care Delivery
Elizabeth McGrath, DNP, AGACNP-BC, AOCNP, ACHPN; Jennifer Willyard; Susan DiStasio; Darcy Kreis; Andrea Bucalleto; Maya Lango
DHMC - Dartmouth Hitchcock
Background
Survivorship care often occurs within a single visit at the end of treatment to discuss a treatment summary and/or survivorship care plan with a patient. With increased understanding of both immediate and long-term effects of cancer and cancer treatment we need to consider support for the patient from the time of diagnosis through the end of the patient’s life. In this quality improvement (QI) initiative, a team of survivorship stakeholders utilized survey and focus group methodology to develop a set of recommendations to support a large, academic medical center in a rural setting to move toward this holistic notion of survivorship care.
Methods
Surveys regarding experience with survivorship care were deployed to oncology providers, allied health providers, and patients and care partners. Surveys were deployed via a web-based survey tool using email groups and lists developed by the oncology department, as well as printed handouts with the survey URL and a QR code available to patients during clinic visits. In addition a focus group was conducted with oncology APPs to discuss findings and to develop recommendations to support survivorship care delivery.
Results
Responses received from 59 patients, 20 care partners, 24 allied health providers, and 19 providers. Results will be discussed with implications for practice reviewed.
Conclusions
There is a need for a shift from survivorship care that’s limited to a dedicated visit type or tied to a survivorship care plan but to one that is woven into all aspects of a patient’s treatment and life beyond treatment.
Assessing Marijuana Use in Cancer Patients Receiving Chemotherapy
Elizabeth McGrath, DNP, AGACNP-BC, AOCNP, ACHPN; Maureen Stannard APRN; Kathleen Broglio APRN; Madelyn Modebeck SN
DHMC - Dartmouth Hitchcock
Background
Use of marijuana among cancer patients is common but tools to evaluate this use are lacking. It is unclear if using marijuana while undergoing chemotherapy is safe and research regarding the risks and benefits are lacking challenging healthcare clinicians in properly educating patients on marijuana use. A retrospective chart review of fifty GI oncology patients was completed to determine how often providers assessed or discussed marijuana use with their patients. Only two charts had marijuana use documented. The goal of this project was to develop a tool to help in the evaluation of marijuana use in cancer patients at the Dartmouth Cancer Center.
Methods
A literature review on marijuana use in cancer patients was conducted using PubMed. Sixteen articles were identified for review. Most articles evaluated use of marijuana for treatment-induced side effects, marijuana versus opioids and placebos, and interactions between chemotherapy agents and marijuana. Evidence that exists focuses on the management of disease and treatment-induced symptoms. These include lack of appetite, weight loss, poor sleep, pain, paresthesia, and neuropathy. All sixteen articles stated there is a need for further research and were hesitant to back positive findings without further studies to replicate findings. Little research has been done on toxicities of marijuana but some studies indicate interactions between marijuana and chemotherapy agents.
Results
A survey was developed to assess marijuana use in GI oncology patients and will be piloted in the Gastrointestinal Oncology clinic. Implementation strategies and results will be discussed.
Conclusions
Clinicians need to screen for marijuana use in cancer patients.
ConnectShareCare: A peer-to-peer online facilitated support network for care partners of people living with serious illness
Elizabeth O'Donnell, MPH1; Beth O'Donnell1; Aricca Van1; Andrea Buccelleto 1;Janet Haines1; Amelia Cullinan1; Matt Wilson1; Alexandra 1; Megan Holthoff1; Dave Gustafson2; Colleen Young3; Andy Williams1;Jen Hinson1; Joel King1; and Kathy Kirkland1
DHMC - Dartmouth Hitchcock1; University of Wisconsin2; Online Community Consultant Toronto, Ontario3Background
Care partners of people with serious illness are often unprepared to manage and cope with the unexpected burden of caregiving and circumstances that arise with disease progression and bereavement. Our objective was to test the feasibility, usability, and utility of an online peer-to-peer facilitated support network for active and bereaved care partners of people with serious illness.
Methods
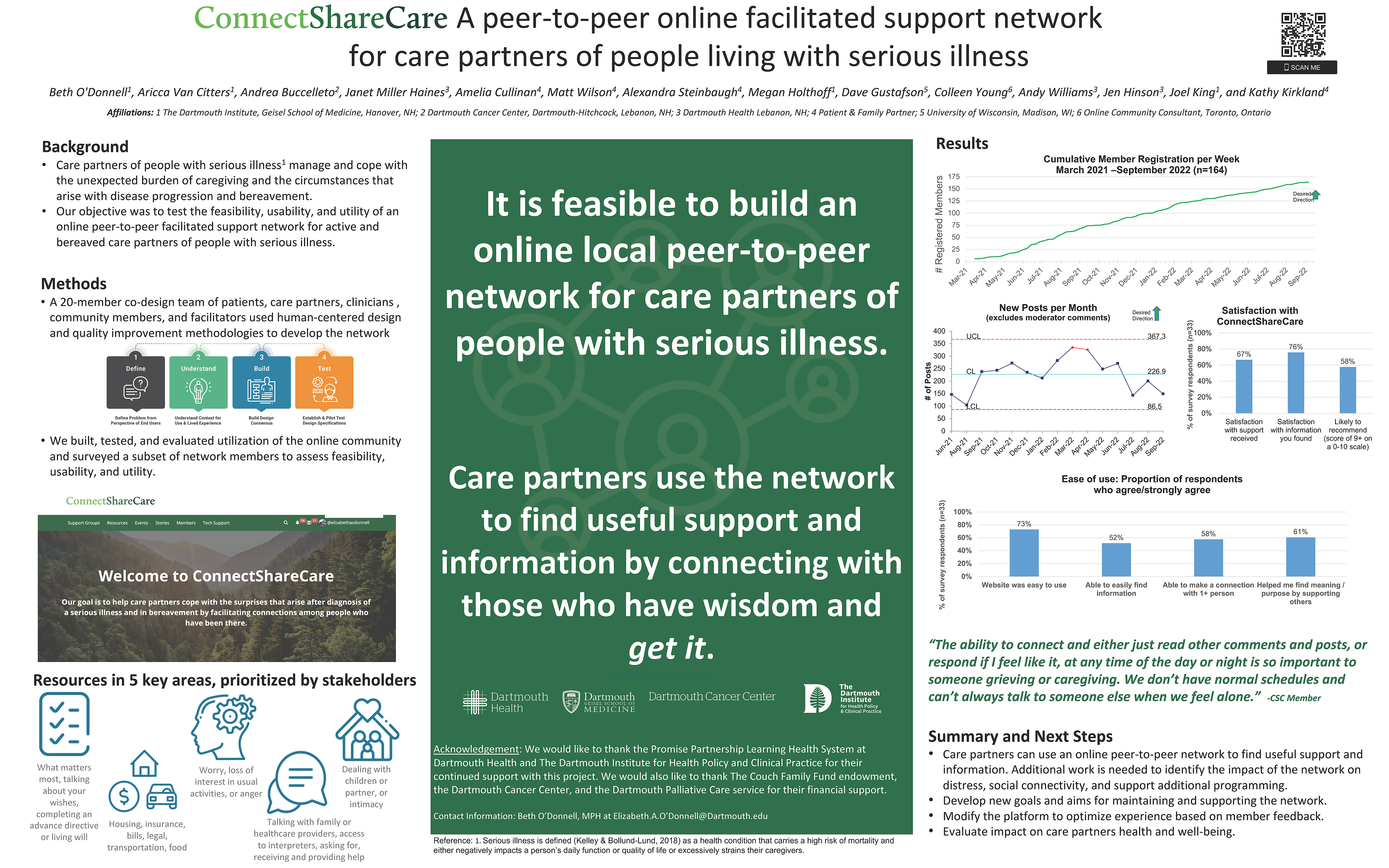
A 20-member co-design team of patients, care partners, healthcare professionals, community members, and researchers used human-centered design to develop an online network for care partners. We evaluated website utilization (May 2021-August 2022) and surveyed a subset of network members to assess feasibility, usability, and utility.
Results
163 members enrolled in the ConnectShareCare network. The monthly average of posts was 230, and half (n=79) of members were active in the last 90 days. Users that completed a research survey (n=33) most commonly accessed the Support Group (n=24, 73%); Private Messaging (n=17, 52%) and Member Stories (n=9, 27%). Nearly half reported visiting weekly (n=16, 48%). Most found the network easy to use (n=24, 73%), and over half could find information (n=17, 52%) and connect with at least one other person (n=19, 58%). Most reported that the network helped them find meaning and purpose by supporting others (n=20, 61%), and were satisfied with support (n=22, 67%) and information (n=25, 76%).
Conclusions
Care partners can use an online peer-to-peer network to find useful support and information. Additional work is needed to identify the impact of the network on distress, social connectivity, and support programming.
Non-Small Cell Lung Cancer: Investigating the Relationship Between Focused Molecular Testing and Next Generation Sequencing in Maine
Anna Pavlova; Jennifer Bourne, MS; Petra Helbig, CCRP; Margaret Chavaree; Leah Graham, PhD;
Jens Rueter, MD
The Jackson Laboratory (Maine Cancer Genomics Initiative)
Background
Focused molecular testing (FMT) or broad panel NGS (bpNGS) can both identify genomic markers in lung cancer. The Maine Cancer Genomics Initiative (MCGI) provided bpNGS testing to 136 patients with non-squamous NSCLC (ns-NSCLC) in Maine between 2017 and 2020. A subset of cases also had FMT done as a routine test in addition to the NGS testing. We investigated the likelihood of identifying an abnormality with either testing modality.
Methods
We analyzed pathology and bpNGS reports for results of 4 molecular markers (EGFR, ALK, ROS1, MET). We used descriptive statistics to compare FMT results to bpNGS testing results.
Results
Testing for at least one FMT marker was completed on 57 cases (42%), bpNGS on 91 cases (67%), 34 cases (25%) had both. EGFRm were detected in 10 (7%), ALKalt in 4 (2.9%), METamp in 3 cases (2.21%), and ROS1alt in 1 case (0.73%). Of these 18 cases, bpNGS detected variants in 10 of these (55%); 7 EGFRm (70%--3 failed), 2 ALKalt (50%, 1 failed, 1 not detected), 1 METamp (1 failed, 1 not detected). The ROS1alt was not detected by bpNGS. 83 bpNGS cases had additional potentially therapeutically actionable variants (median 2 per case, range: 1-8).
Conclusions
In this small cohort, mutations in EGFR are readily detected, but actionable aberrations in ALK, ROS1 or MET were missed by bpNGS. Additional genomic variants not associated with FDA approvals may be detected by bpNGS, creating clinical trial and off-label treatment options. Consideration should be given to performing both testing modalities.
The Effect of Extended Panel Molecular Testing on Treatment Decisions for Patients with Lung, Breast, and Colon Cancer
Jacqueline Pesco1, OMS II; Topalovic2 S, B.S.; Thomas3 CA, MD
1University of New England College of Osteopathic Medicine; 2Dana Farber Cancer Institute; 3New England Cancer Specialists
Background
Extended Panel Molecular Testing (EPMT) is increasingly utilized for patients with advanced malignancies. We aimed to determine the impact of EPMT results on the treatment of patients with metastatic breast, colon, and lung cancer by assessing the number and potential actionability of EPMT and their effect on patients' treatment selection. We also examined EPMT test utilization (tissue versus circulating tumor DNA testing).
Methods
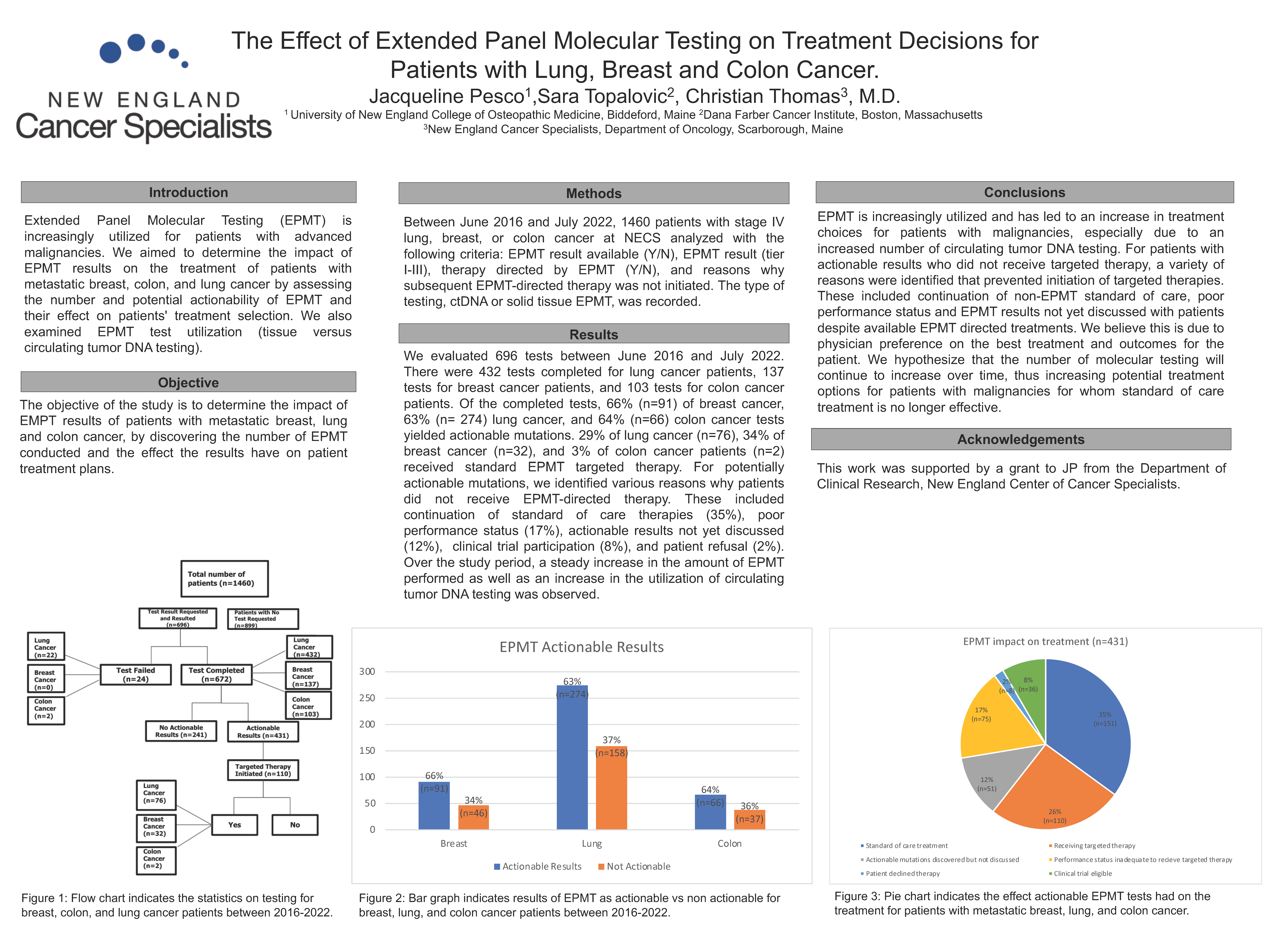
Between 2016 and 2022, 1460 patients with stage IV lung, breast, or colon cancer at NECS were evaluated for ctDNA or solid tissue EPMT results (tier I-III), and reasons to not initiate therapy.
Results
We evaluated 696 tests between 2016 and 2022, (432 lung, 137 breast, 103 colon cancer). Actionable mutations; 66% (n=91, breast cancer), 63% (lung cancer, n= 274), 64% (colon cancer, n=66). Targeted therapy received: 80 lung (29%), 31 breast (34%), colon 2 (3%). Reasons to not receive EPMT-directed therapy: standard therapy (34%), clinical trial (8.5%), poor performance status (18%), patient refusal (2%). EPMT testing and the percentage of circulating tumor DNA testing increased over time.
Conclusions
EPMT is increasingly utilized especially due to an increased circulating tumor DNA testing. We identified reasons why patients with actionable results did not receive targeted therapy. We hypothesize that the number of molecular testing will continue to increase over time, thus increasing potential treatment options for patients with malignancies for whom standard of care treatment is no longer effective.
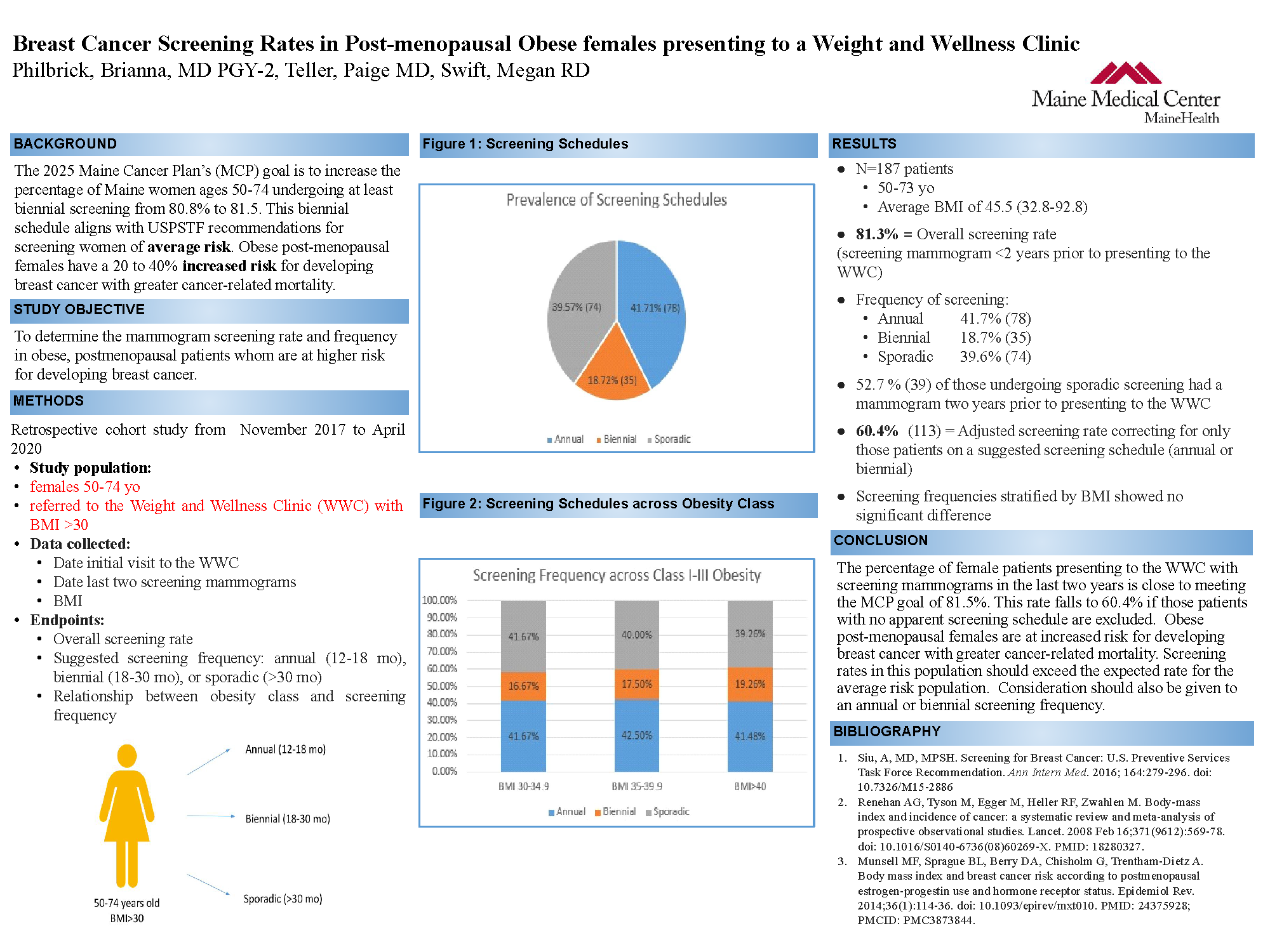
Breast Cancer Screening Patterns in Obese Postmenopausal Women presenting to Weight and Wellness Clinic
Brianna Philbrick; Paige Teller, MD; Megan Swift, RD
Maine Medical Center
Background
The Maine Cancer Plan’s (MCP) goal is to increase the percentage of Maine women ages 50-74 undergoing at least biennial screening from 80.8% to 81.5% by 2025. Obese postmenopausal females have a 20 to 40% increased risk for developing breast cancer with greater cancer-related mortality. We sought to determine if the mammogram screening rate and screening frequency in obese, postmenopausal patients is appropriate in this higher risk population.
Methods
Chart review was performed on a sample of female patients aged 50-74 presenting on referral to the Weight and Wellness Clinic (WCC) for obesity (BMI>30).
Dates of last two screening mammograms were used to determine screening rate and suggested screening schedule: annual (12-18 mo), biennial (18-30 mo), or sporadic (>30 mo).
Results
The total number of patients out of 187 that had a screening mammogram within two years was 152 (81.3%). The number of patients undergoing a suggested annual, biennial, and sporadic screening schedule were 78 (41.7%), 35 (18.7%), and 74 (39.6%) respectively (Figure 1). Of the patients that underwent sporadic screening, 39 (52.7%) had a mammogram two years prior to presenting to the WWC. If the overall screening rate was corrected to account for only those patients on a suggested screening schedule, the rate would be adjusted to 113 (60.4%).
Conclusions
Obese, postmenopausal females are not meeting mammogram screening goals. Efforts must be made to improve screening rates in this high risk population. Rates should exceed that expected for the average risk population, giving consideration to an annual screening frequency.
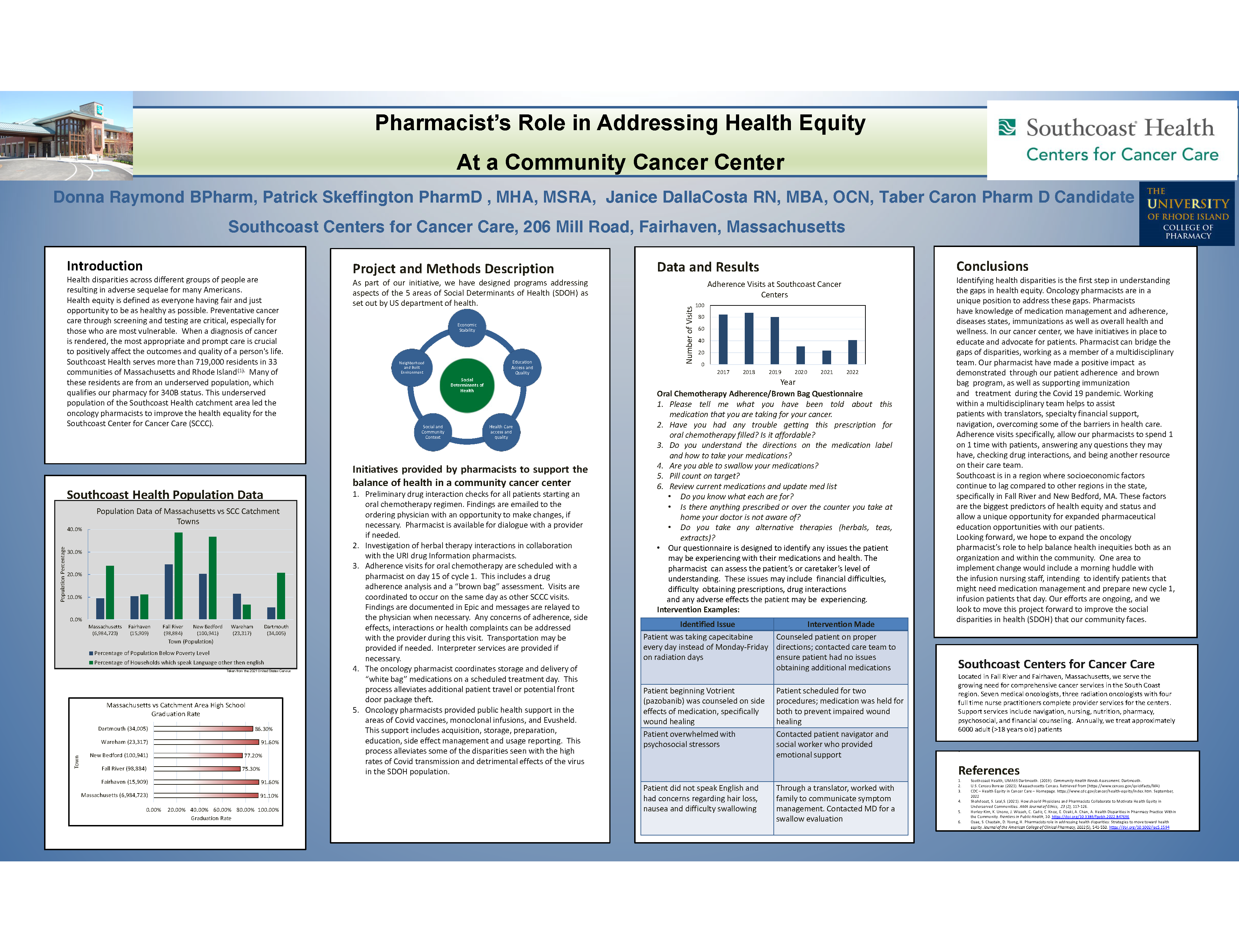
Pharmacists’ role in addressing health inequity at a community cancer center
Donna Raymond, RPH; Janice DallaCosta RN,MBA,OCN; Patrick Skeffington, PharmD, MHA, MSRA Team Lead Oncology Pharmacy
South Coast Centers for cancer care
Background
Health equity is defined as everyone having fair and just opportunity to be as healthy as possible. The CDC states, “That health equity is achieved when every person has the opportunity to attain their full health potential “. Our pharmacists at Southcoast Center for Cancer Care (SCCC) have made efforts to improve quality of care and improve health equality for our patients.
Methods
A retrospective review of Southcoast’s oncology pharmacy programs included, oral chemotherapy adherence visits, prescribed and over the counter medication interactions as well as herbal products interaction checks. We provide support to the covid infusion and vaccine clinics and facilitation of “white bagging “. Review of the SCCC catchment area specifically determined that we care for a population of underserved patients.
Results
Retrospective review of our clinical pharmacy programs demonstrated our efforts to reduce health inequity within our cancer center catchment area and has resulted with positive patient outcomes. The pharmacists at SCCC show support to the cancer center with an “all hands on deck “approach offering additional programs needed for a population that is typically underserved.
Conclusions
Oncology Pharmacists at SCCC offer necessary clinical support while also addressing health inequity. The efforts are ongoing and we look to move this project forward to improve the social disparities of health (SDOH) in our community.
Onsite Rural Cancer Center Fresh Food Pantry Pilot Underscores Depth of Need
Catherine Reed, MSW, LICSW; Chelsey R Canavan, MSPH; Greg A Norman, MS; Elizabeth B McGrath, DNP APRN, AGACNP-BC, AOCNP, ACHPN; John M Hill Jr., MD; Kenneth R Meehan, MD
Dartmouth Cancer Center
Background
At a rural academic medical center in New England loss of employment due to cancer, not medical bills, has proven to be the greatest cause of patients’ financial toxicity and food insecurity. Loss of income, exacerbated by the COVID-19 pandemic and 40-year record high inflation is forcing our patients to have to choose between gas to get to their appointments, and food and nutrition to support health and cancer treatment tolerance.
Methods
We instituted a Social Determinants of Health (SDoH) screening at the patients’ first provider visit and included food insecurity using the validated Hunger Vital Signs metric. An EPIC Best Practice Alert notified the care team in real-time of patients needing food. A local community food pantry partner delivered fresh produce, dairy and staples to our food pantry. A LICSW staff member took tailored food orders, packed bags, and delivered food directly to patients’ cars.
Results
SDoH screener results show that 10-11% of our patients are food insecure. In the initial six month period we provided 67 food insecure patients with two weeks of tailored food for their households (175 deliveries and 425 bags). Demographics, costs and implementation of this project will be discussed.
Conclusions
An onsite, rural cancer center food pantry benefits food insecure patients who are exhausted, nutritionally at risk, and financially compromised, especially during a pandemic. We need to expand our food pantry operations from 3 to 10 clinical oncology groups to provide vital up-front cancer treatment to all our under-resourced patients.
A Patient with Small-cell Lung Carcinoma and Long-term Survival: Using a Checkpoint Inhibitor to Enhance Antitumor Immunity
Ivy Riano M.D.,1 Katherine Rose N.P., Keisuke Shirai, M.D.1
1 Dartmouth Cancer Center, Geisel School of Medicine Dartmouth College, Lebanon, NH
Background: Small cell lung cancer (SCLC) has a high risk of early hematogenous and lymphatic dissemination. Despite response rates of 60-65% to platinum chemotherapy, limited progress has been made in the latest years, and outcomes remain poor, with a median overall survival of approximately 10 months.
Methods: Herein, we report a case of a patient with limited-stage SCLC treated with chemotherapy and immunotherapy with 60-month survival.
Results: A 69-year-old Caucasian male presented with cough in the spring of 2017. A CT scan showed a right upper lobe mass with mediastinal lymphadenopathy. Biopsy from subcarinal lymph node was consistent with SCLC. He received 4-cycle of cisplatin and etoposide as well as prophylactic intracranial irradiation. His subsequent CT chest did not show evidence of recurrent disease. On March 2019, he presented with recurrent disease, and was treated with 4-cycle of cisplatin/etoposide with addition of atezolizumab until May 2020, when he was found with hypermetabolic mediastinal/pericardial lymph nodes and pericardial metastasis. Due to the progression of his disease, lubernectine was used, then docetaxel, and on January 2021 he was started on pembrolizumab. To date, patient remains on treatment with single agent pembrolizumab with his latest CT scan without disease progression.
Conclusions: Our patient is one of the few cases with long survival reported in the literature. SCLC has a high mutation rate, which suggests that these tumors may be immunogenic and could respond to immune-checkpoint inhibitors. Adding immunotherapy to chemotherapy may enhance antitumor immunity; more research is needed to confirm this hypothesis.
Cherenkov Imaging to Verify Radiation Treatment Delivery in Breast Cancer
Melanie Rose1; D. A. Alexander2; M. Jermyn2,3;S. M. Decker2; G. S. Gill1; D. J. Gladstone2; B. W. Pogue2,4; C. R. Thomas Jr1,5; and L. A. Jarvis15
1Dartmouth-Hitchcock Medical Center; 2Thayer School of Engineering; 3DoseOptics, LLC; 4University of Wisconsin; 5Geisel School of Medicine
Background
Cherenkov imaging can record beam shapes on patients, assessing positioning and dose accuracy. We compared predicted surface dose to Cherenkov images to assess treatment accuracy quantitatively and qualitatively, testing this new methodology.
Methods
IRB approved data collection was completed for all patients receiving breast radiation therapy with 6X and/or 10X beams. Predicted surface dose was obtained by using CT simulation images to create a 3D surface dose at a specified Hounsfield unit threshold on -200 HU. For each fraction, Dice coefficient (% similarity), MDC (mean distance to conformity), and incident coefficient (confidence of signal outside the planned area) were measured.
Results
Cherenkov images were collected between May-June 2021, taken from 204 fractions and surface planning volumes from 21 plans. MDCs were found to average 3.4mm (std dev 1.8mm), DICE coefficient averaged 93.7% (std dev 2.8%), and mean incident coefficient averaged 0.4% (std dev 0.6%). Visually, the plan overlay was non-identical to the imaged Cherenkov dose in 7 patients noted laterally, medially, and the superior edges of the planning fields.
Conclusions
Advancements in Cherenkov imaging allows us to compare fractions with planned surface dose using qualitative and quantitative data.
Creating a Single Point of Referral and Standardized Care Pathway for Melanoma Patients to Improve Access to care
Sydney Rubin-Fitzgerald, RN; Bailey Hanson, BA- DH; Andrew Loehrer, MD, MPH- DH
DHMC - Norris Cotton Cancer Center
Background
Access to care is often a challenge for healthcare organizations and is often complex to solve. In this quality improvement (QI) initiative, we deployed the Lean Six Sigma approach to develop a two-pronged method that reduced the median time to first visit for new melanoma patients.
Methods
The Define, Measure, Analyze, Improve, and Control (DMAIC) processes was used to address access to care after the healthcare organization set a goal that 55% of new specialty care patients be seen within 10 days. The lack of a well-defined and standardized referral workflow for melanoma patients caused delays in patient care as only 31% of incoming referrals were seen within 10 days, and the median number of days to first visit was 15, or 50% over the organizational standard.
Results
Analysis found considerable waste in the existing referral system as untrained staff made referral and routing decisions causing rework, inappropriate visits and delays. Two interventions were deployed to address the identified waste, a centralized referral point and a referral questionnaire were developed by an interdisciplinary team to guide where routine cases should be seen. After the intervention 54% of newly referred patients were seen within 10 days.
Conclusions
Improvements in access can be achieved by adopting standard work that is targeted to reduce waste in the referral workflow. The model developed by this QI initiative is easily replicable and may be especially useful for interdisciplinary teams who provide care to complex diseases.
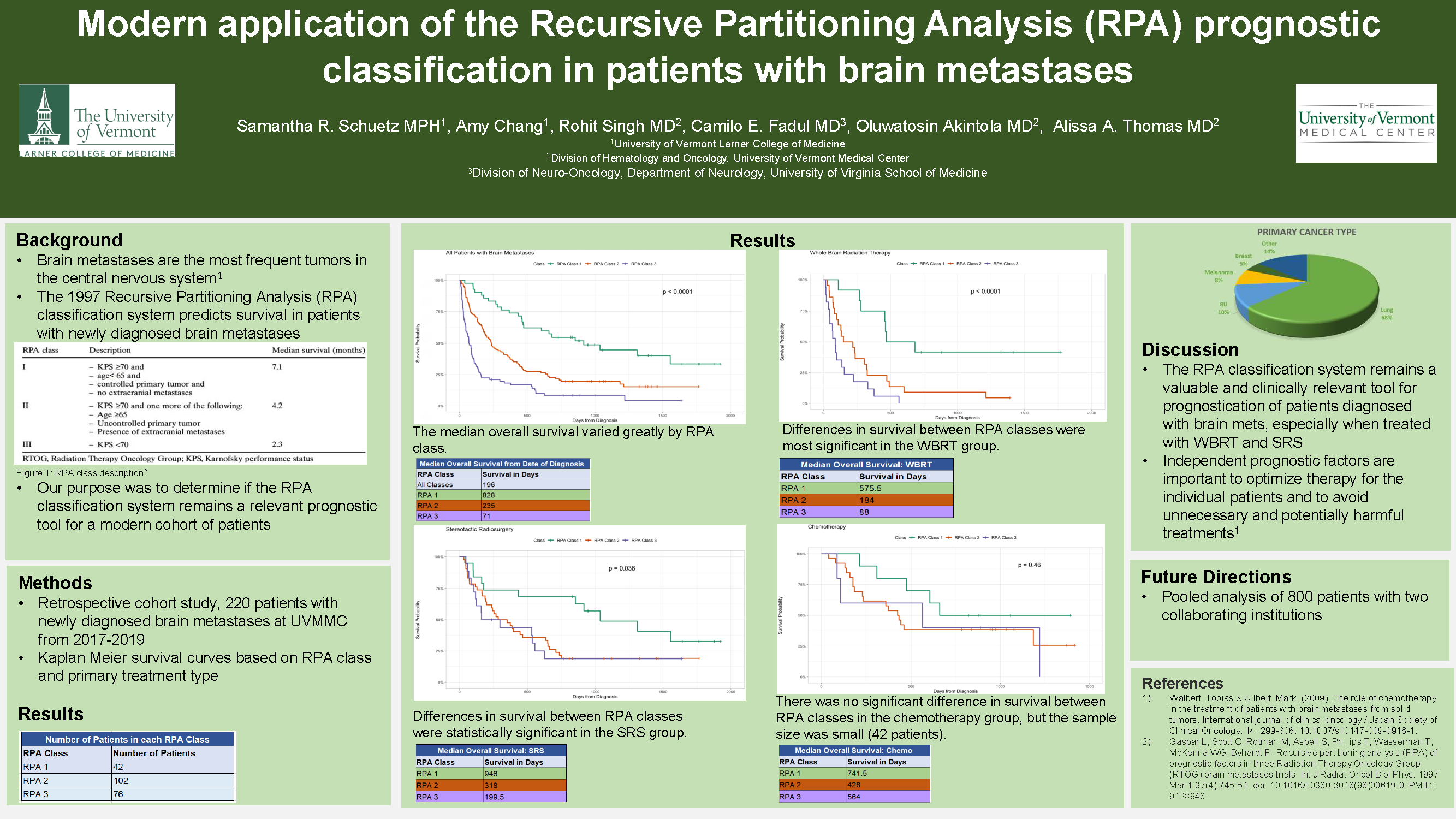
Modern Application of the Recursive Partitioning Analysis (RPA) Classification in the Prognosis of Patients with Brain Metastases
Samantha Schuetz, MPH; Amy Chang; Rohit Singh; Camilo Fadul; Oluwatosin Akintola; Alissa A. Thomas
Larner College of Medicine at University of Vermont
Background
The 1997 RPA classification system predicts survival in patients with newly diagnosed brain metastases (BMets). Our purpose was to determine if this classification system is still relevant to a modern cohort of patients.
Methods
We performed a retrospective cohort study in patients newly diagnosed with BMets at the University of Vermont Medical Center from 2017-2019. We analyzed patient survival data based on RPA class by primary treatment type (whole brain radiation (WBRT), stereotactic radiosurgery (SRS), or chemotherapy). We created Kaplan-Meier survival curves for patients based on RPA class and treatment type.
Results
We identified 220 patients with BMets with ≥ 6 months of follow-up. The median overall survival (OS) from BMets diagnosis for all patients was 196 days. When further analyzed by RPA class, the median overall survival from time of diagnosis varied greatly (RPA 1 survival=769 days, RPA 2 survival=233 days, RPA 3 survival=71 days). Median OS by RPA class differed by primary treatment group. There was statistical significance in the survival curves based on RPA class and primary treatment modality of WBRT and SRS. Median OS for patients treated with WBRT in RPA 1 was 469 days, RPA 2 was 184 days, and RPA 3 was 78.5 days. Median OS for patients treated with SRS in RPA 1 was 885.5 days, RPA 2 was 269 days, and RPA 3 was 199.5 days.
Conclusions
The RPA classification system remains a valuable and clinically relevant tool for prognostication, especially for patients treated with WBRT and SRS.
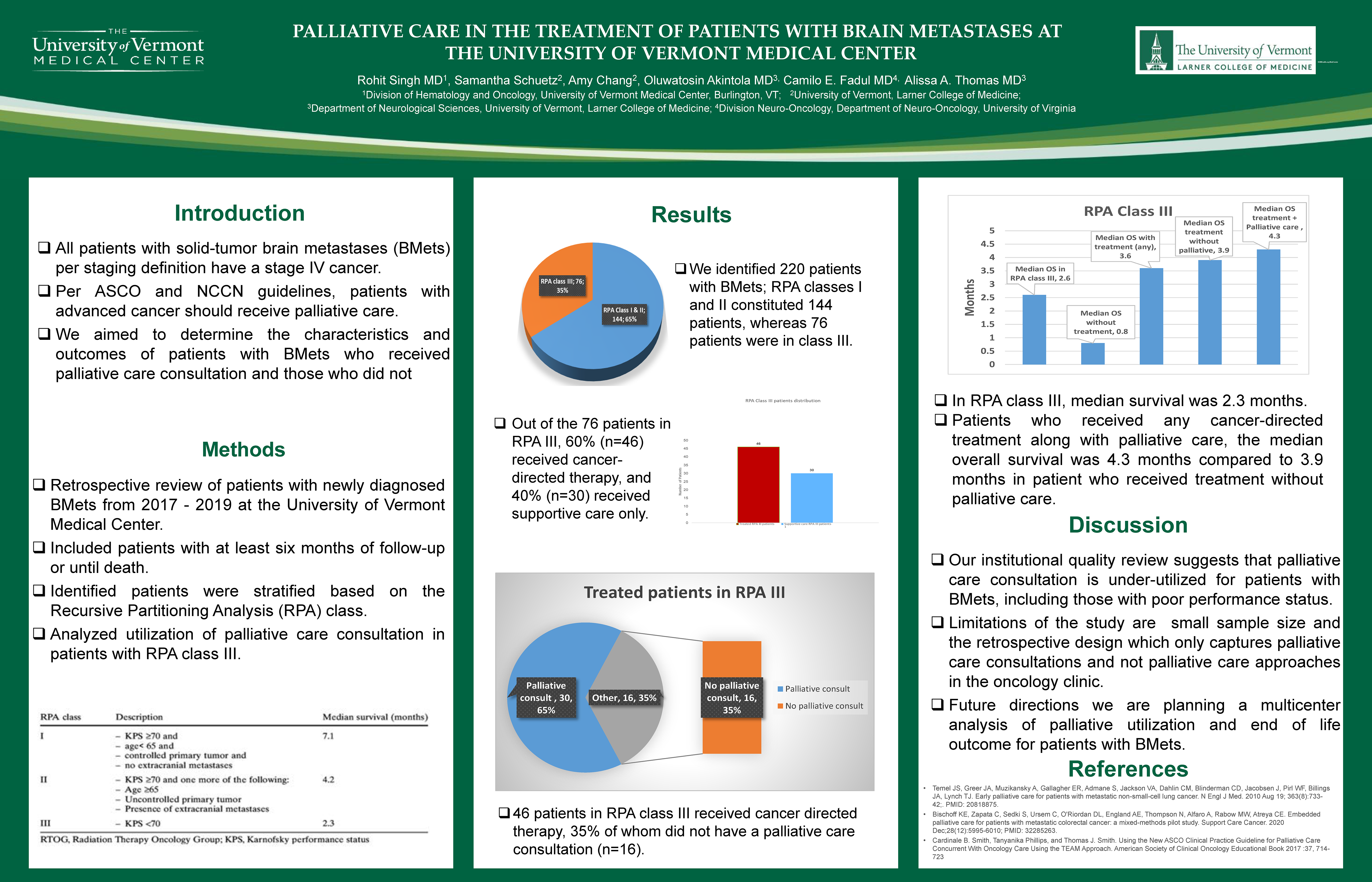
Palliative Care in the Treatment of Patients with Brain Metastases at the University of Vermont Medical Center
Rohit Singh1, MD; Samantha Schuetz1; Amy Chang; Oluwatosin Akintola1 MD; Camilo E. Fadul2,MD; Alissa A. Thomas1, MD
1University of Vermont Medical Center; 2University of Virginia
Background
All patients with solid-tumor brain metastases (BMets) have stage 4 cancer. Per ASCO guidelines, all patients with advanced cancer should receive palliative care. We aimed to determine the characteristics and outlook of patients with BMets depending on their palliative care consultation.
Methods
We retrospectively reviewed patients with newly diagnosed BMets from 2017-2019 at the University of Vermont and included patients with at least six months of follow-up or until death. We analyzed data regarding the utilization of palliative care in patients with poor performance status (RPA class III).
Results
We identified 220 patients with BMets; RPA classes I and II constituted 144 patients, whereas 76 patients were in class III (KPS <70). Of the entire cohort, 128 (58%) were evaluated by palliative care. In RPA class III, 70% of patients (n=53/76) received palliative care. In the RPA III cohort, 84% of patients (n=64) received cancer-directed therapy, and 16% (n=12) received supportive care only. The median survival for all patients with BMets was 6.5 months; however, for patients with RPA III, the median survival was 2.3 months. In RPA III, in patients who received any cancer-directed treatment along with palliative care, the median survival was 140 days compared to 29 days in the supportive care-only cohort.
Conclusions
Our institutional quality review suggests that palliative care consultation is under-utilized for patients with BMets, including those with poor performance status. Multidisciplinary care involving palliative care must be integrated into treating all patients with metastatic disease, especially BMets.
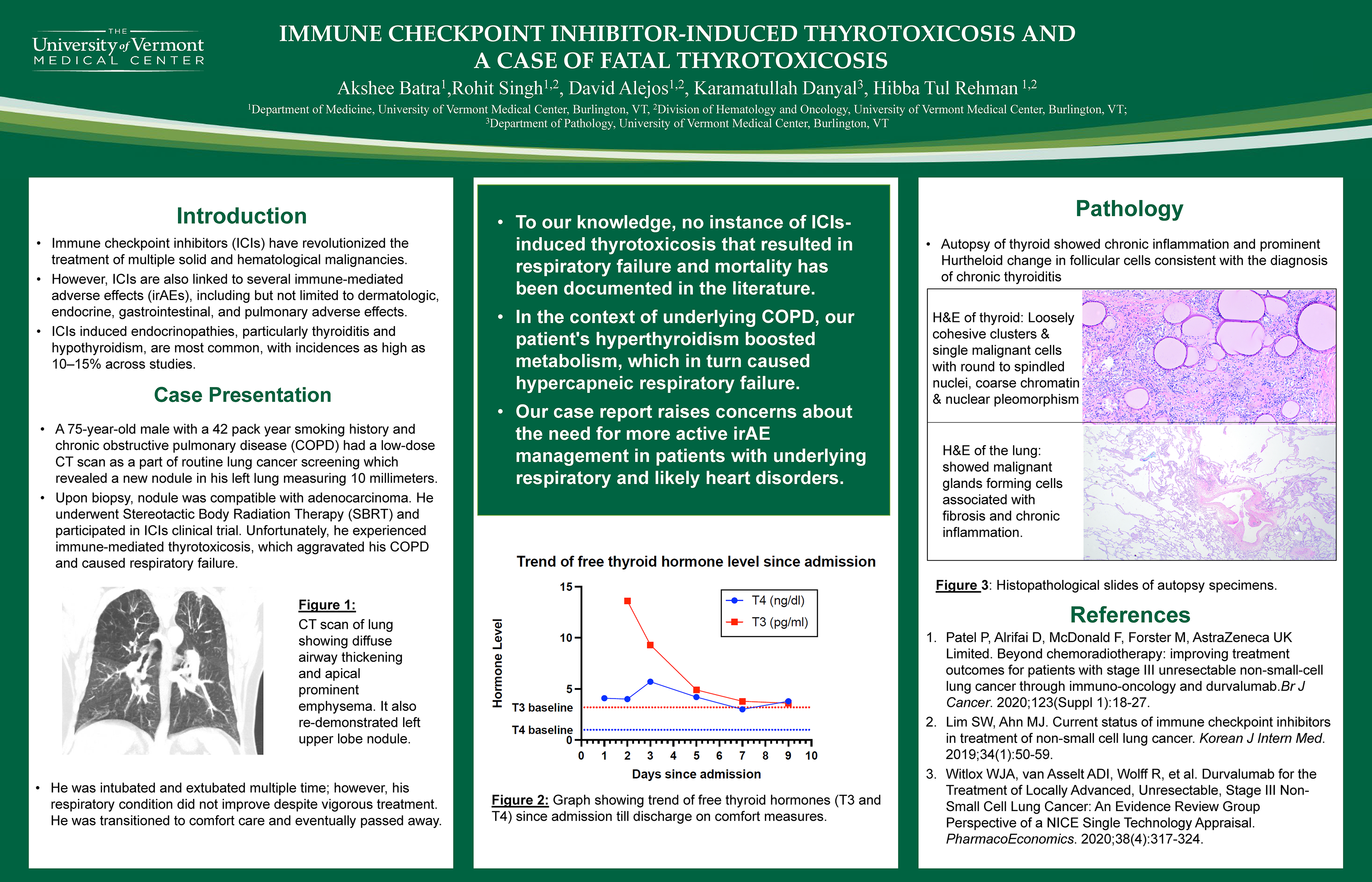
Immune checkpoint inhibitor-induced thyrotoxicosis and a case of fatal thyrotoxicosis
Rohit Singh, David Alejos MD; Akshee Batra MD; Hibba tul Rehman MD
University of Vermont Medical Center
Background
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of multiple solid and hematological malignancies. However, ICIs are also linked to several immune-mediated adverse effects (irAEs). ICIs are also associated with a wide variety of irAEs, including but not limited to dermatologic, endocrine, gastrointestinal, and pulmonary adverse effects. ICIs induced endocrinopathies, particularly thyroiditis and hypothyroidism, are most common, with incidences as high as 10–15% across studies.
Methods
Our patient, a 75-year-old man, had a long smoking history and chronic obstructive pulmonary disease (COPD). He had a low-dose computed tomography (CT) scan performed as part of a routine lung cancer screening. It revealed a new nodule in his left lung measuring 10 millimeters, which was compatible with adenocarcinoma upon biopsy. He underwent stereotactic body radiation therapy (SBRT), and as part of his therapy, he also participated in a clinical trial and received ICIs. Unfortunately, he experienced immune-mediated thyrotoxicosis, which aggravated his COPD and caused respiratory failure. He was intubated and extubated multiple times for respiratory support; however, his respiratory condition did not improve despite vigorous treatment. He was transitioned to comfort care and eventually passed away.
Results
To our knowledge, no instance of ICIs-induced thyrotoxicosis that resulted in respiratory failure and mortality has been documented in the literature.
Conclusions
In the context of underlying COPD, our patient's hyperthyroidism boosted metabolism, which in turn caused hypercapnic respiratory failure. This raises concerns about the need for more active irAE management in patients with underlying respiratory and likely heart disorders.
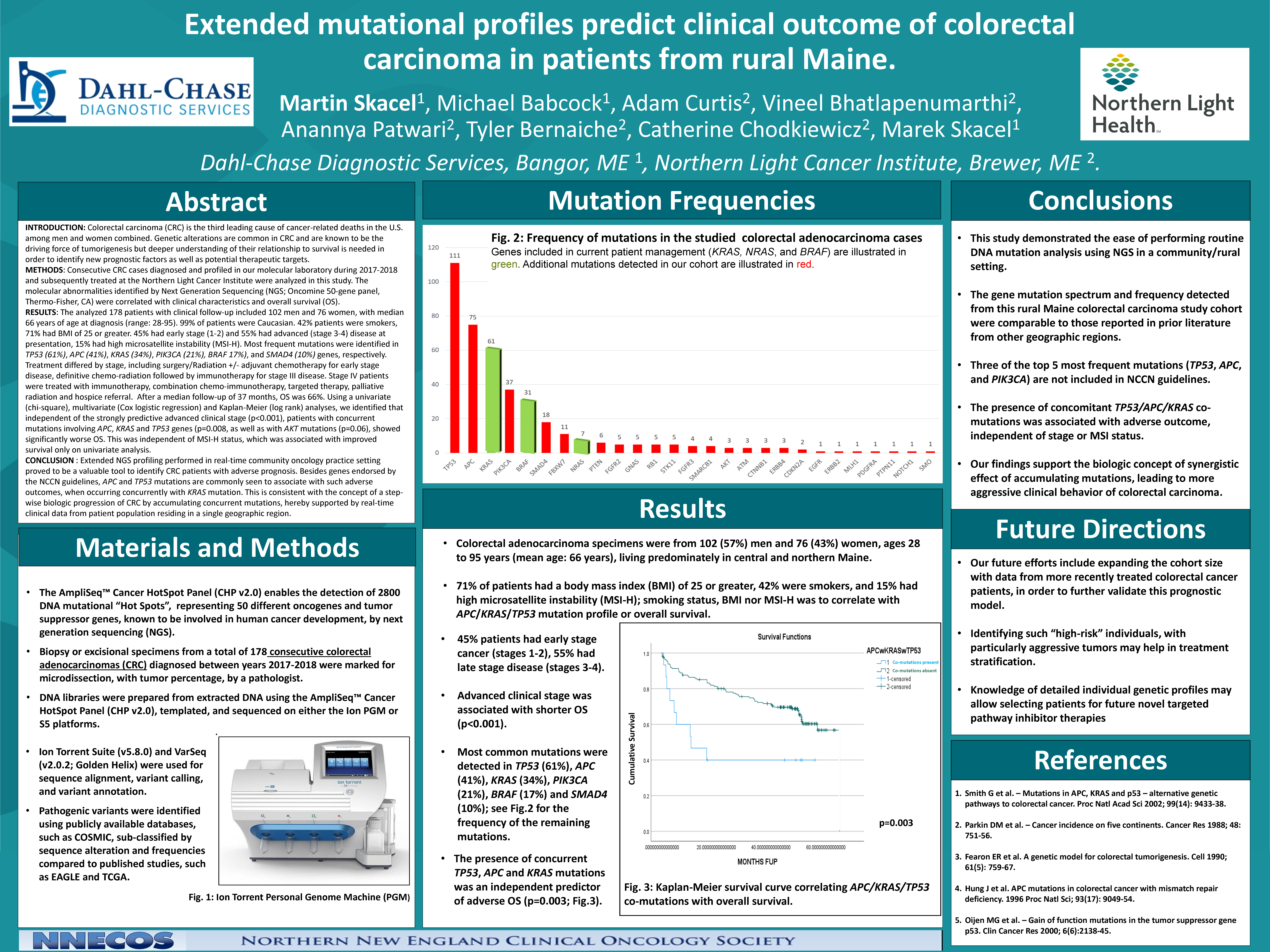
Extended mutational profiles predict clinical outcome of colorectal carcinoma (CRC) in patients from rural Maine
Martin Skacel; Michael Babcock; Adam Curtis; Vineel Bhatlapenumarthi; Anannya Patwari; Tyler Bernaiche; Catherine Chodkiewicz; Marek Skacel
Dahl-Chase Pathology; Northern Light Cancer Institute
Background
CRC is the third leading cause of cancer-related deaths in the U.S. Genetic alterations are the driving force of CRC tumorigenesis but deeper understanding of their relationship to survival is needed.
Methods
Consecutive CRCs diagnosed in 2017-2018 were analyzed by Next Generation Sequencing (NGS; Oncomine, Thermo-Fisher, CA) and findings correlated with clinical characteristics and overall survival (OS).
Results
178 patients with follow-up included 102 men and 76 women, median age of 66 years (range: 28-95), 42% smokers, 71% of BMI 25 or greater, 18% rectal, 39% right-sided, 45% early stage (I/II), 55% advanced stage (III/IV), 15% of MSI-H. Mutations occurred most frequently in TP53 (61%), APC (41%), KRAS (34%), PIK3CA (21%), BRAF 17%), and SMAD4 (10%) genes. Treatment based on NCCN guidelines included surgery +/- adjuvant chemotherapy for early stages, chemo-radiation, immunotherapy/chemo-immunotherapy, targeted therapy or palliative therapy for advanced stages. At median follow-up of 37 months, OS was 66%. Advanced clinical stage (p<0.001), concurrent mutations in APC, KRAS and TP53 genes (p=0.003), and rare AKT mutations (p=0.06), showed independent association with adverse OS on multivariate analysis. MSI-H showed improved survival on univariate analysis only.
Conclusions
Extended NGS deployed in routine community oncology practice identifies CRC patients with adverse outcome. Outside of NCCN-endorsed testing, APC and TP53 mutations are associated with decreased survival, when occurring concurrently with KRAS mutation. This indicates cumulative CRC mutational progression, supported by real-time patient data from a single geographic region.
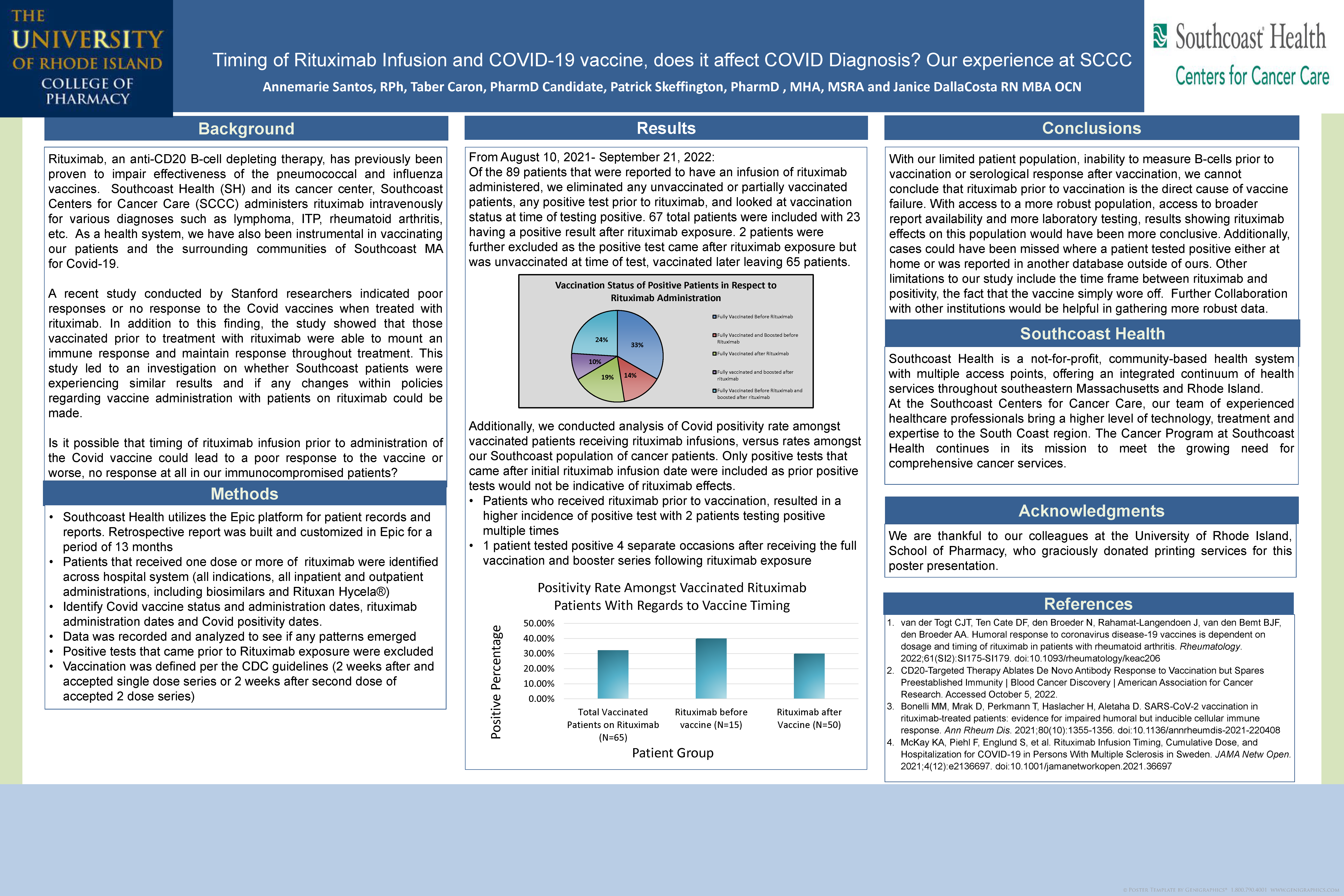
Timing of rituximab infusion and COVID-19 vaccine does it affect COVID diagnosis our experience at SCCC
Patrick Skeffington, PharmD, MHA, MSRA; Annemarie Santos, RPh; Janice DallaCosta RN, MBA; OCN/clinical Nurse Manager
Southcoast Centers for Cancer Care
Background
Review timing of administrations of the COVID-19 vaccine and rituximab infusions in relation to a COVID diagnosis rituximab, an anti-CD20 B-cell depleting therapy, has previously been proven to impair the effectiveness and immune response of the pneumococcal and influenza vaccines. Southcoast Health administers rituximab intravenously for different diagnoses in various outpatient settings
Methods
- Retrospective report was built and customized using the EPIC database
- Rituximab patients identified (all indications, inpatient, outpatient doses)
- Rituximab doses given, COVID vaccine dates reported and diagnoses of COVID were looked at to see if any patterns emerged
Results
Southcoast Health identified 88 patients who received at least one dose of Rituximab from August 10, 2021 through August 10, 2022:
15 patients had a COVID diagnosis:
- Five were partial vaccinated
- Three no vaccine doses
- Seven fully vaccinated patients
1. 2 had COVID prior to getting vaccinated
2. 2 received at least one dose of rituximab prior to vaccine dose
3. 3 received NO rituximab until after 2 doses of vaccines (ideal)
Conclusions
SCCC sample size not large enough to show any trends with respect to rituximab and COVID vaccine and diagnosis of COVID. Large studies have concluded rituximab can cause inhibition of the B cell response to the mRNA COVID vaccine with timing of administration playing a role in this mechanism. Patients who had rituximab dose 6-12 months prior to mRNA vaccine may not respond to the vaccine increasing risk of COVID.
Pulmonary artery intimal sarcoma: a rare cause of intracardiac masses
Zachary Smith, BS; Brendan Campbell, MD; Rohit Singh, MD; Naomi Hodde, MD
University of Vermont
Background
Pulmonary artery intimal sarcoma (PAIS) is an exceedingly rare malignant neoplasm arising from subendothelial pluripotent cells within the pulmonary artery intimal lining. To date, roughly 300 cases of PAIS have been described. Given the lack of standardized treatment guidelines these patients often have very poor survival outcomes.
Methods
A 60-year-old male with a past medical history of tobacco use presented to the emergency department with two weeks of persistent abdominal pain, and subjective fever. Computerized tomography (CT) abdomen/pelvis with contrast revealed multiple intra-abdominal abscesses along with multiple hypo-attenuating poorly enhancing pancreatic and adrenal masses. He was admitted for management of intra-abdominal abscesses with IV antibiotics.
Results
Subsequent CT chest angiogram of the chest revealed multiple intracardiac masses resulting in severe stenosis of the pulmonary artery trunk with bilateral pulmonary embolism. The differential included primary cardiac angiosarcoma, pancreatic neuroendocrine malignancy, melanoma, lymphoma, sarcoidosis, tuberculosis, blastomycosis, histoplasmosis, and aspergillosis.
He developed clinical signs of right heart failure over the next two weeks. The patient and family decided to pursue comfort measures only care due to the advanced nature of the disease. The patient was discharged to home hospice and died three weeks later, and a final diagnosis of PAIS was made at the autopsy.
Conclusions
PAIS imaging findings can be similar to acute or chronic pulmonary thromboembolism or intracardiac thrombus and is often misdiagnosed in the early stages of the disease. This case illustrates a complicated presentation of this extraordinarily rare malignancy.
Significant Financial Burden Facing Patients Receiving Cellular Immunotherapy at a Rural NCI-Designated Cancer Center*
Kristian Sogaard, MAT; Catherine M Reed MSW, LICSW; John M Hill Jr MD; Kenneth R Meehan MD
Geisel School of Medicine at Dartmouth
Background
There is limited research evaluating financial toxicity in patients receiving cellular immunotherapy (CIT); we thus sought to characterize the severity and distribution of economic burden within this group at our rural, NCI-designated, comprehensive cancer center.
Methods
Between 2017-2022, a social worker assessed the needs of 201 patients undergoing CIT by administering a psychosocial assessment. Further follow-up was performed to elaborate individual financial needs. Categories of financial burden assessed were food, housing, lodging, transportation, utilities, and hospital financial assistance. The reference period for needs assessment spanned 18 months, between 6 months pre-CIT and 12 months post-CIT.
Results
Within the 18-month reporting period, forty-four (22%) individuals required financial assistance. The average amount of total support per patient was $3148.62. Categories of financial assistance and amount awarded (average) per recipient included: outside grants- $2505.88; transportation- $415.88; food- $406.46. Additionally, financial assistance per patient pre-pandemic was $2843.73 (mean) compared to $3482.28 during COVID, a 22.5% increase.
Conclusions
Financial toxicity among CIT patients remains an under-appreciated and poorly studied area in this high-risk patient population. Basic needs, particularly transportation, food, and heating fuel can be barriers to CIT and directly impact quality of life and survival after transplant. Such challenges are exacerbated by a rural, New England environment and, often, long-distance commutes. The dramatic increase in financial toxicity during a global pandemic and record-high inflation further underscores the need for additional research and support for this vulnerable patient population.
Affiliation - Blood and Marrow Transplant Program, Section of Hematology/Oncology, Dartmouth-Hitchcock Medical Center, Lebanon, NH
Evaluating clinician emotion regulation during a serious illness conversation in oncology using multimodal assessment: a pilot study
Garrett T. Wasp, MD MPH1; Satveer Kaur, PhD1; Eric Anderson, PhD2 ; Maxwell T. Vergo, MD1; Julia Chelen, PhD1; Tor Tosteson, ScD3; Paul J. Barr, PhD MSC1; Amber E Barnato, MD MPH MS1;1Dartmouth-Hitchcock Medical Center (DHMC); 2MaineHealth Institute for Research; 3Tufts University School of Medicine
Background
Clinician emotion regulation (ER), self and the patient’s, likely moderates successful prognostic discussions with patients, yet challenges around its evaluation limit its investigation. We performed a pilot study to develop and assess an experimental framework that uses multimodal assessment (self-report, observer, and biometric data) to measure clinician ER during a simulated, serious illness conversation (SIC).
Methods
We developed our experimental framework in four steps: 1) drafted a patient case and assessment framework; 2) refined the data collection strategy; 3) trained our actor; and 4) iteratively piloted the case. For the assessment, we conducted a cross-sectional, case series pilot study with physicians trained in SIC to assess feasibility and acceptability. To characterize clinician ER strategies, we analyzed the visit dialogue, physician interviews performed while watching the visit back, and physician SIC documentation. We used a hybrid approach of inductive and deductive coding and theme development based on preexisting ER theory.
Results
The development phase yielded two major modifications: 1) abandoned use of AppleWatch; and 2) used telehealth context. We approached 12 physicians and 11 enrolled: 5 were medical oncologists, and 6 specialty palliative care physicians. All participants completed all study survey items. The results of our three HRV sensors were as follows: Empatica E4 and Polar H10 met our pre-specified HRV collection in all 11 resting tasks and SIC encounters, and the Scoche R24 the benchmark in 7/11 resting tasks and 4/11 of simulated encounters.
Conclusions
The use of multimodal assessment of clinician ER in a simulated, telehealth SIC visit was acceptable and feasible.
Spreading Routine Serious Illness Conversations at a Single Cancer Center Using a Multidisciplinary and Patient-Family Advisory Approach
Garrett T. Wasp, MD MPH; Karen Guo, DMD; Maxwell T. Vergo, MD; Matthew Wilson, MD; Andy Williams; James J. Perry, OTR, CHT, LSSBB; Megan M. Holthoff, MSHS; Madge E. Buus-Frank DNP, APRN-BC, FAAN; Amelia M. Cullinan, MD
Dartmouth-Hitchcock Medical Center (DHMC)
Background
We describe the spreading of a multidisciplinary and patient-family advisor (PFA) quality improvement (QI) initiative to conduct routine serious illness conservations at a NCI-designated comprehensive cancer center.
Methods
This study describes the second phase of a learning health system initiative to conduct routine SIC for all seriously ill patients with cancer. Prior work included defining patient eligibility, and electronic health record (EHR) modifications. Phase II can be categorized into three steps: a) increasing communication coach and PFA capacity; b) refining EHR tools and c) adoption of the Model for Improvement. For the first three months, each team engaged in preparatory work. In the last six months, teams met weekly to set goals, and conducted iterative PDSAs.
Results
Over nine months (3/1/2021 to 12/31/21), four teams screened 510 patients with cancer, identified 272 (53%) patients as eligible for SIC, and 178 (65%) of those eligible had a documented SIC from a baseline of 0%. The breakdown of this combined SIC patient count by clinician author is as follows: team A 40 (22%); team B 45 (25%); team C 9 (5%); team D 14 (8%) and specialty palliative care 70 (39%). Each team set modest, initial SIC documentation goals (range 5-15%), and attainment of first SIC documentation goal varied (range 2 to 4 months). Noted challenges with PFA recruitment and retention included: lengthy recruitment, integration into clinical teams, and resistance to change by teams.
Conclusions
The multidisciplinary approach, inclusive of specialty palliative care, increased SIC documentation. PFA involvement met with challenges and yielded mixed results.


%20Final.png)