2020 Accepted Spring Abstracts
* indicates selected for podium presentation
A retrospective Chart Review Analysis of Therapy related AML. A Single Center Experience.
Vineel Bhatlapenumarthi1, Anannya Patwari1, Adam Curtis2, Sheila Rajan2, Antoine J Harb2
1 Northern Light Eastern Maine Medical Center, 2 Northern Light Cancer Institute
Background: Therapy-related acute myeloid leukemia (t-AML) is a group of hematological neoplasms that occurs in patients who were exposed to cytotoxic chemotherapy and or radiation. It is usually associated complex cytogenetics and poor prognosis.
Methods: We conducted a retrospective chart review of adult AML patients from 2010 till 2019. Among this cohort, we identified who previously received chemotherapy and or radiation for a different pathology/disease and subsequently developed t-AML.
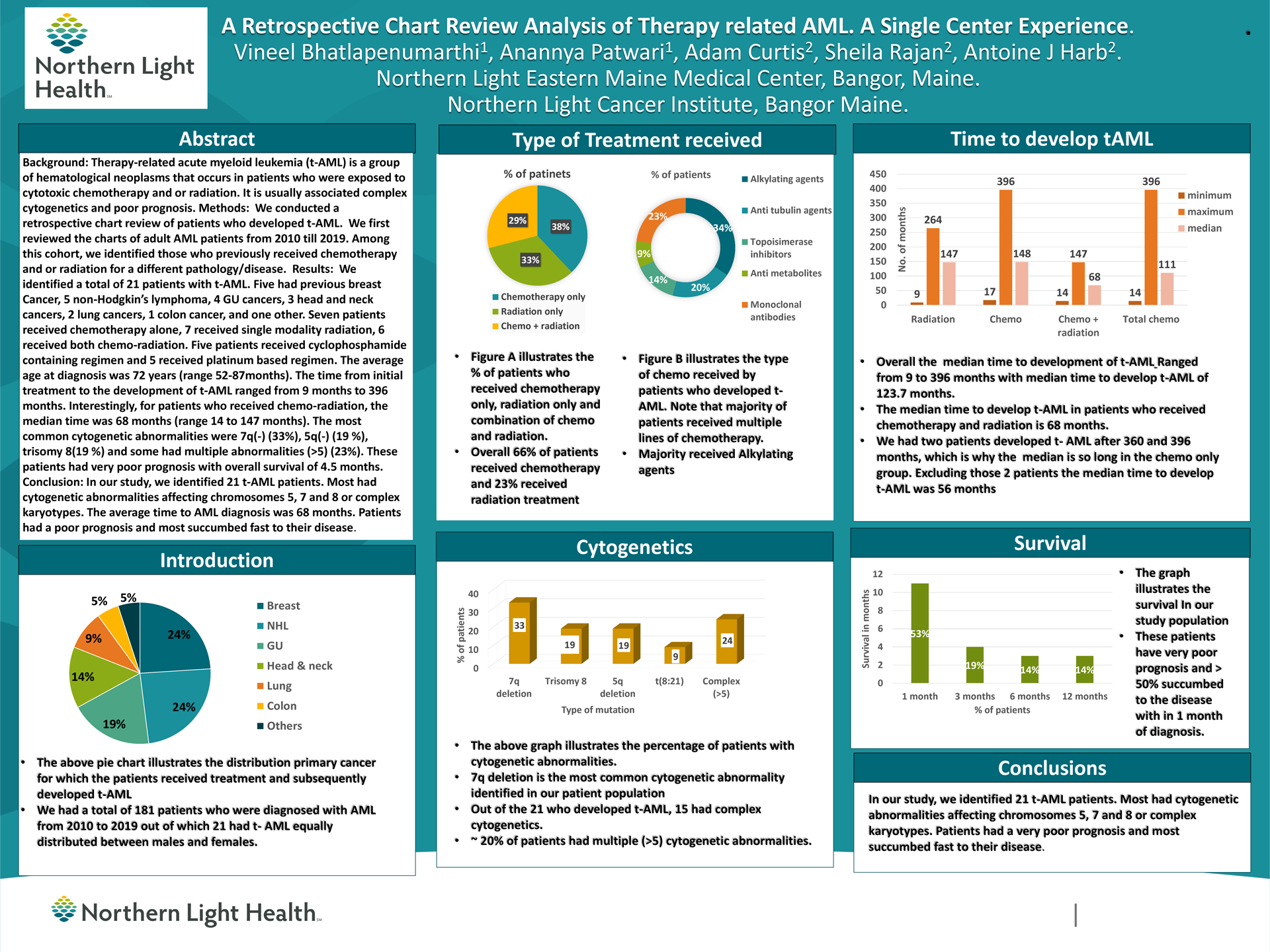
Results: We identified a total of 21 patients with t-AML. 5 had previous breast Cancer, 5 non-Hodgkin’s lymphoma, 4 GU cancers, 3 head and neck cancers, 2 lung cancers, 1 colon cancer, and one other. 7 patients received chemotherapy alone, 7 received single modality radiation, 6 received both chemo-radiation. 7 patients received cyclophosphamide containing regimen and 5 received platinum-based regimen.
The average age at diagnosis was 72 years (range 52-87months). The time from initial treatment to the development of t-AML ranged from 9 months to 396 months. Interestingly, for patients who received chemo-radiation, the median time was 68 months (range 14 to 147 months).
The most common cytogenetic abnormalities were 7q(-) (33%), 5q(-) (19 %), trisomy 8(19 %) and some had multiple abnormalities (>5) (23%).
These patients had very poor prognosis with overall survival of 4.5 months.
Conclusions: In our study, we identified 21 t-AML patients. Most had cytogenetic abnormalities affecting chromosomes 5, 7 and 8 or complex karyotypes. The average time to AML diagnosis was 68 months. Patients had a poor prognosis and most succumbed fast to their disease.
Colorectal Cancer (CRC) in Patients from Rural Maine: Correlation of Molecular Profiles with Clinical Outcome.
Vineel Bhatlapenumarthi1, Anannya Patwari1, Michael J Babcock2, Adam Curtis1, Antoine Harb1, Kristi Mitchell2, Laura Skacel2, Maria Michailidou1, Catherine Chodkiewicz1, Marek Skacel21 Northern-Light Cancer Institute, Brewer, Maine, 2 Northern-Light Eastern Maine Medical center, Bangor, Maine. 3 Dahl-Chase Pathology Associates, Bangor, Maine.
Background: The National Comprehensive Cancer Network (NCCN) recommends molecular testing for BRAF, KRAS, NRAS gene mutations and microsatellite instability (MSI) in CRC. In this study, mutational findings were correlated with clinical outcome in CRC patients from rural Maine.
Methods: Mutational profiles of 192 CRC patients diagnosed in 2017 and 2018 were analyzed by next-generation sequencing (NGS). Mutational analysis included BRAF (exon 15), and RAS family biomarkers (KRAS/NRAS exons 2-4). MSI testing was performed using the MSI Analysis System. SPSS was used to perform Chi-square and Kaplan-Meier (log rank) survival analysis.
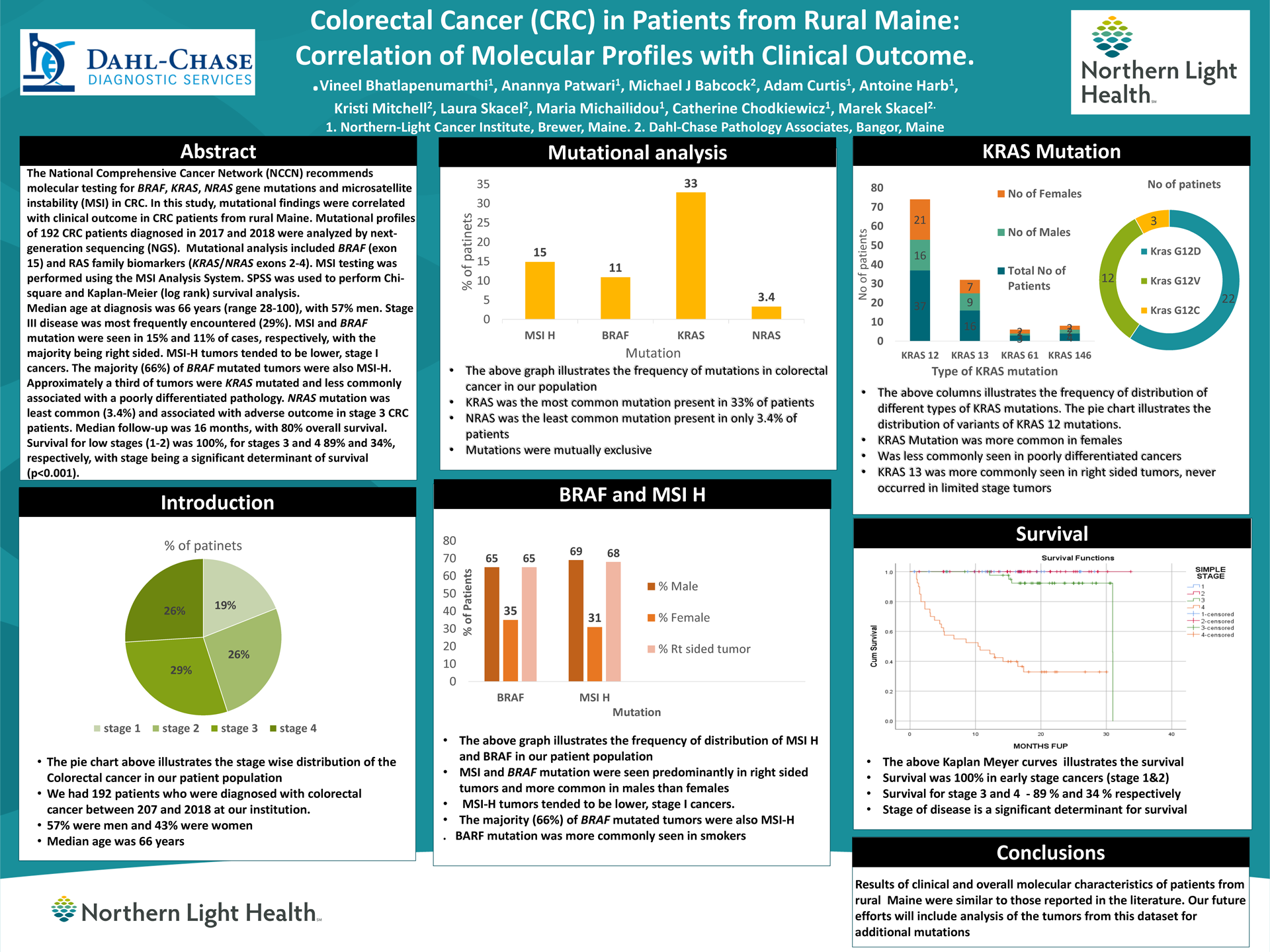
Results: Median age at diagnosis was 66 years (range 28-100), with 57% men and 43% women. Stage III disease was most frequently encountered (29%). MSI and BRAF mutation were seen in 15% and 11% of cases, respectively, with the majority being right sided. MSI-H tumors tended to be lower, stage I cancers. The majority (66%) of BRAF mutated tumors were also MSI-H. Approximately a third of tumors were KRAS mutated and less commonly associated with a poorly differentiated pathology. NRAS mutation was least common (3.4%). Median follow up was 16 months with 80% overall survival. Survival for low stage (1-2) was 100% for stages 3 and 4, 89 and 34 % respectively, with stage being a significant determinant of survival (p<0.001).
Conclusions: The clinical and molecular characteristics of rural Maine CRC patients were similar to those reported in the literature. In future studies, we intend to analyze the additional mutations outside of the NCCN panel.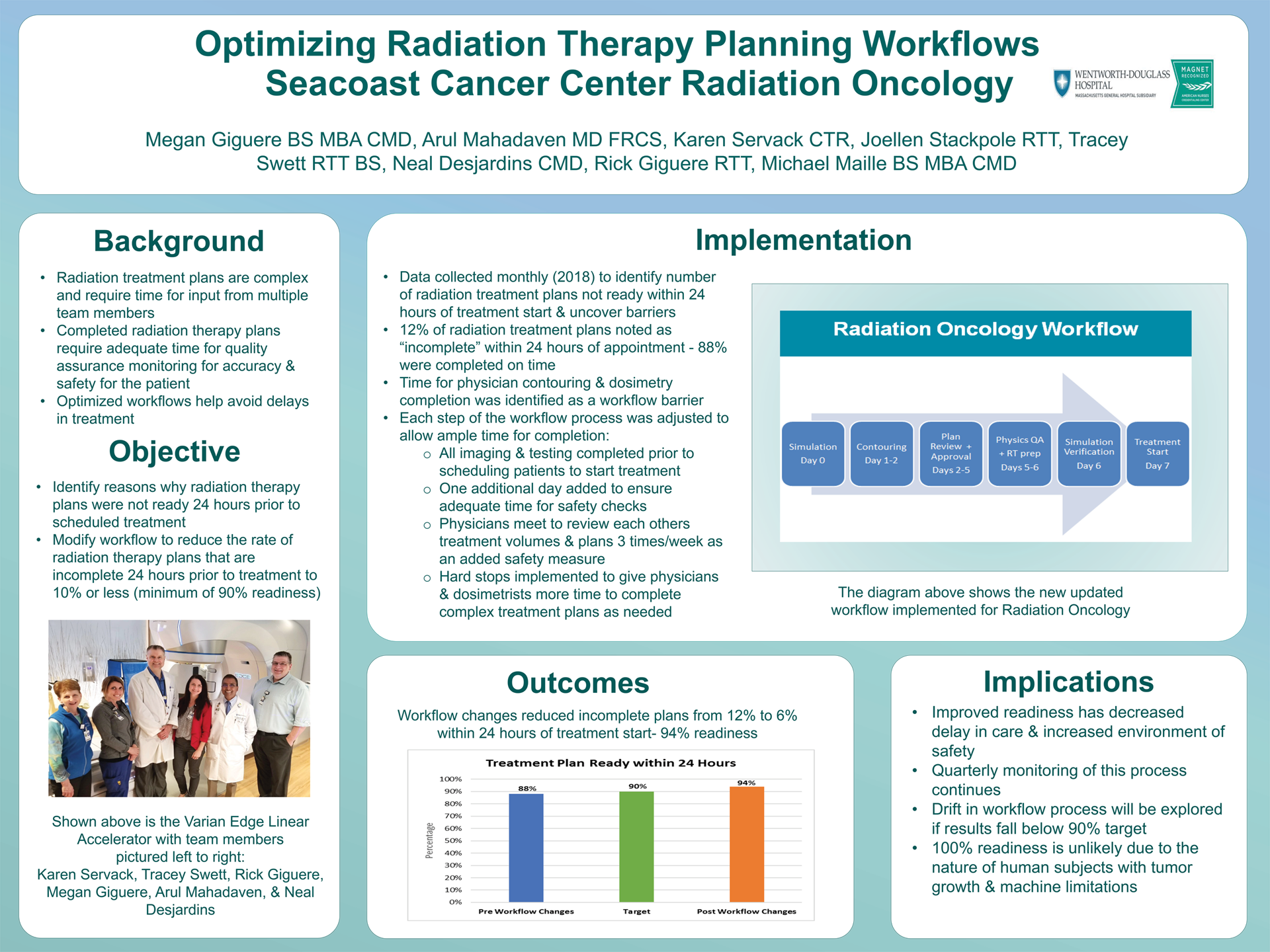
Optimizing Radiation Therapy Planning Workflows
Megan Giguere, CMD, Arul Mahadevan MD FRCS,Tracey Swett RTT BS, Rick Giguere RTT, Joellen Stackpole RTT, Karen Servack CTR, Neal Desjardins CMD, Micheal Maille BS MBA CMD
Wentworth Douglass Hospital
Background: Radiation treatment plans are complex and require time for input from multiple team members to provide optimal accuracy. “Optimizing Radiation Therapy Planning Workflows,” was a project designed to reduce the incidence of radiation therapy plans that were incomplete 24 hours prior to the patients scheduled session.
Methods: The team collected and analyzed quarterly data from 2018 to determine how many radiation treatment plans were not finalized within 24 hours of the session. It was noted that only 88% of cases had complete treatment plans in place within 24 hours of the scheduled radiation session. Changes subsequently were made in the workflow: the first change was to not allow patients to be scheduled until all imaging/testing was completed; the second was to adjust the workflow so that each step has enough time to be completed; lastly, was to schedule patients out one additional day. Further examination of the data revealed that most the dosimetry plans were late due to the physician not contouring in a timely manner and dosimetry not finishing the plan in the allotted timeframe. This led to the implementation of hard stops in the workflow for when dosimetry and physicians need more time to complete their work.
Results: The workflow process changes resulted in 94% of plans being completed 24 hours prior to start of scheduled session.
Conclusions: Data collection continues with monitoring for treatment plan readiness within 24 hours. A drift in process will be explored if results trend from current state of 94%.
An Interprofessional Hospice Training Pilot Program for Allied Health Students*
Amy Litterini, PT, DPT, Dahne Yaitanes
University Of New EnglandBackground: Hospice and palliative care training and exposure are critical components within the curricula of allied health care education majors. Developing opportunities for students through community collaborations is necessary to address this need. The purpose of this pilot initiative was to assess the feasibility of a formal program collaboration between an academic institution and a local hospice provider.
Methods: The University of New England partnered with Compassus Hospice for the “Celebrate Life” pilot program in spring, 2019. Evening trainings, with dinner provided, accommodated the students’ course schedules. Six healthcare major students (physical therapy, occupational therapy and social work) completed formal hospice volunteer training and were matched with an experienced volunteer or faculty mentor to provide 6-8 companionship visits with a patient enrolled in hospice to develop a Life Book. Books were presented to the patients at a final visit celebration. Pre- and post-surveys were completed electronically to assess attitudes, motivations and comfort level for working with patients receiving hospice.
Results: Six student-mentor pairs worked with six patients receiving hospice for companionship and the creation and presentation of a Life Book. Four students completed both pre- and post-survey data. Comfort level working with patients on hospice increased following participation in the pilot program.
Conclusions: This project demonstrated the feasibility of this pilot program collaboration with positive outcomes observed for the participants. Additional training, experiential opportunities with virtual reality scenarios, and observations in other hospice settings, are being explored to further enhance the hospice training experience for students.
Cancer Mortality: Sources of Misclassification in Death Certificates*
Alexandra Morgan1, Judy R Rees2, MD, PhD, Sylvia Guerra1, MTS, Valeria Luna3
1 Geisel School of Medicine, 2 Associate Professor of Epidemiology, Geisel School of Medicine, 3 New Hampshire State Cancer Registry
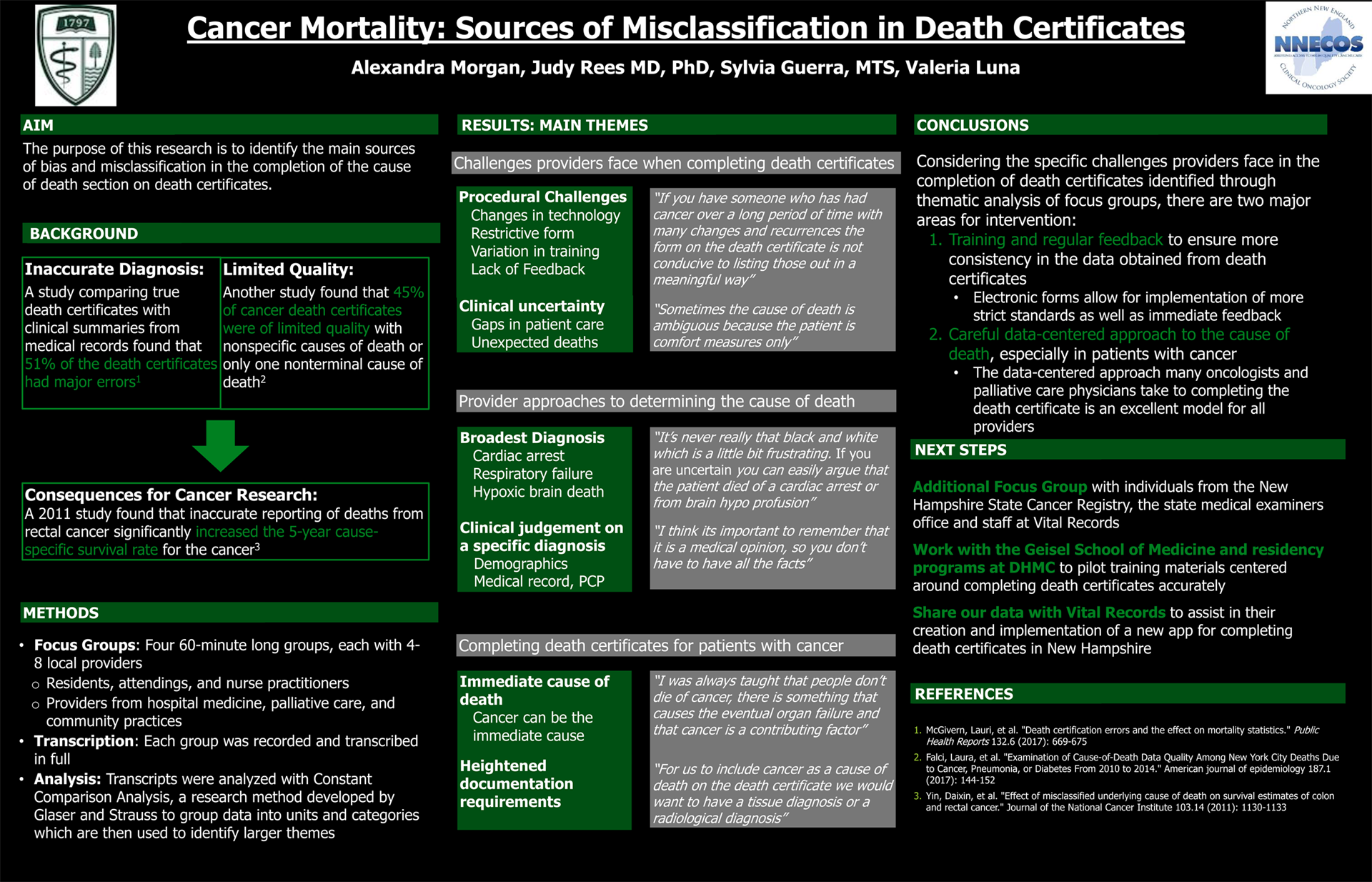
Background: Death certificates are important documents that contribute to mortality statistics and help shape health care policy. Although several aspects of the death certificate are standardized, the completion of the cause of death section varies widely between practitioners. This variation has led to significant misclassification and error in the cause of death section on death certificates which in turn has impacted the statistics used to monitor the etiology, prevention and survivorship of cancer.
Methods: Four focus groups were conducted, each with 4-6 providers from internal medicine, oncology and palliative care practicing in hospital and community settings. Transcripts from the groups were analyzed using Constant Comparison Analysis to group data into themes.
Results: Two main themes arose around the challenges providers face when completing death certificates; procedural challenges and clinical uncertainty. When faced with patients who die from an unknown medical cause, two main themes arose in the approach that providers took to determine the cause of death; listing broad but accurate diagnoses and making a clinical judgement on a narrower diagnosis. In the context of patients with cancer, two interesting challenges emerged; determining the immediate cause of death and the perception of heightened documentation requirements especially among palliative care providers and oncologists.
Conclusions: Improving the accuracy of death certificates is imperative to the accurate documentation of cancer incidence and mortality. Efforts to improve documentation must take into consideration specific challenges faced by providers in determining causes of death and the most common tactics used when this is not precisely known.
Serious Neurological Side Effects in Patients Receiving Immune Checkpoint Inhibitors: a Single Center Experience in Rural Maine*
Anannya Patwari1, Vineel Bhatlapenumarthi1, Antoine Harb2
1 Northern Light Eastern Maine Medical center, Bangor, Maine, 2 Northern Light Cancer Institute, Brewer, Maine
Background: Serious neurological side effects in patients with cancer who are receiving Immune checkpoint inhibitors (ICI) are relatively uncommon with incidence of less than 1%. Transient motor and sensory neuropathies are the most common.
Methods: We conducted a retrospective chart review analysis of patients with cancer who received Nivolumab, Pembrolizumab, Atezolizumab or Durvalumab from 2015 till early 2019, at our institution. Among the patients who received immunotherapy, we identified those who developed serious neurological toxicities requiring treatment interruption or discontinuation.
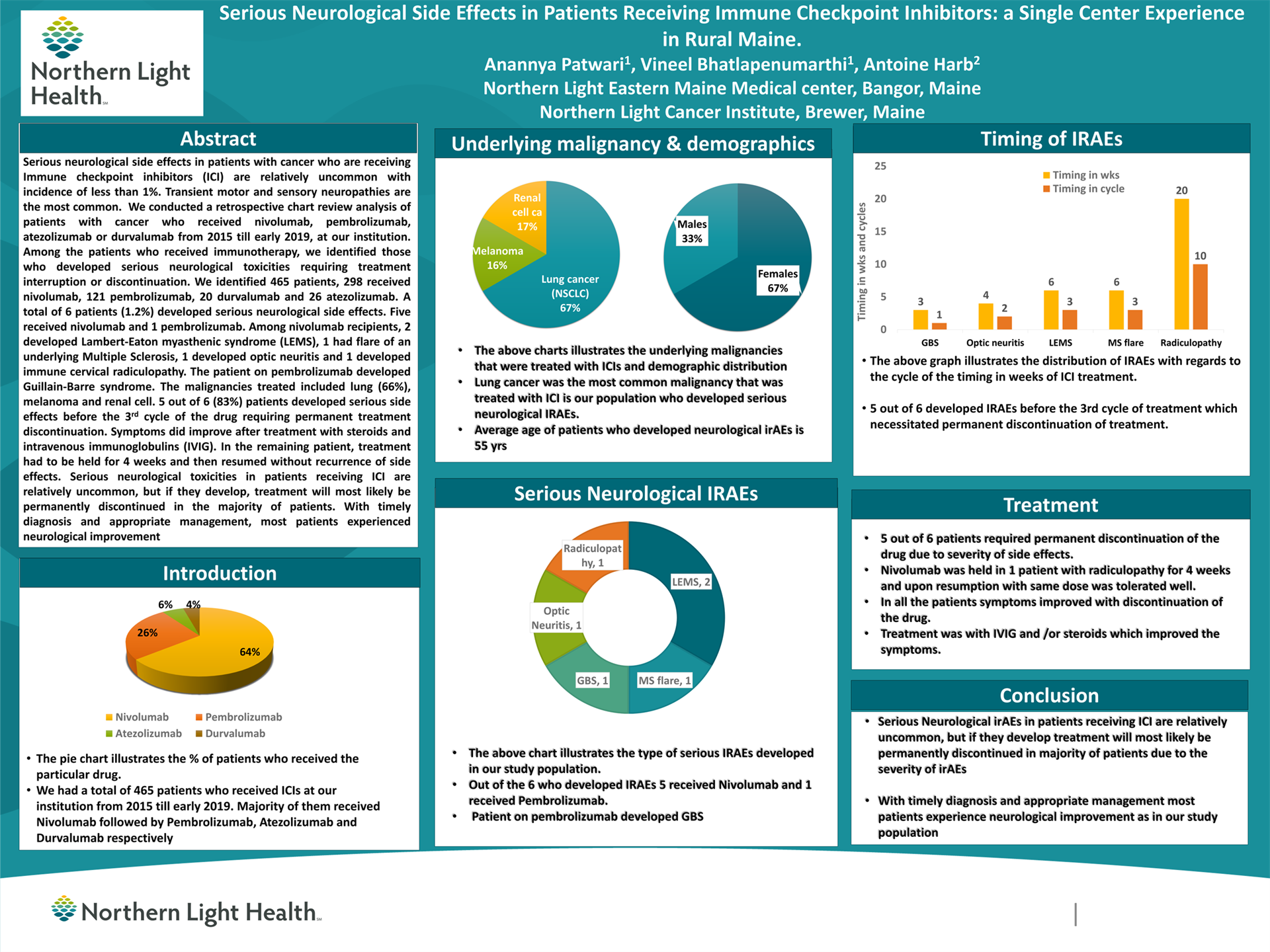
Results: We identified 465 patients, 298 received Nivolumab, 121 Pembrolizumab, 20 Durvalumab and 26 Atezolizumab. A total of 6 patients (1.2%) developed serious neurological side effects. Five received Nivolumab and 1 Pembrolizumab. Among Nivolumab recipients, 2 developed Lambert-Eaton myasthenic syndrome (LEMS), 1 had flare of an underlying Multiple Sclerosis, 1 developed optic neuritis and 1 developed immune cervical radiculopathy. The patient on pembrolizumab developed Guillain-Barre syndrome. The malignancies treated included lung (66%), melanoma and renal cell.
Five out of 6 (83%) patients developed serious side effects before the 3rd cycle of the drug requiring permanent treatment discontinuation. Symptoms did improve after treatment with steroids and intravenous immunoglobulins (IVIG). In the remaining patient, treatment had to be held for 4 weeks and then resumed without recurrence of side effects.
Conclusions: Serious neurological toxicities in patients receiving ICI are relatively uncommon, but if they develop, treatment will most likely be permanently discontinued in the majority of patients. With timely diagnosis and appropriate management, most patients experienced neurological improvement.
Tolerability of Immune Checkpoint inhibitors in Patients with Underlying Autoimmune Conditions: A Single Center Experience in Rural Maine
Anannya Patwari1, Vineel Bhatlapenumarthi1, Antoine Harb2
1 Northern Light Eastern Maine Medical Center, Bangor, Maine, 2 Northern Light Cancer Institute, Brewer, Maine.
Background: Cancer Immunotherapy is frequently associated with immune-mediated side effects, and is often not recommended in patients with underlying autoimmune conditions. The exact incidence of these side effects is largely unknown, as patients with preexisting autoimmune conditions were largely excluded from clinical trials.
Methods: We conducted a retrospective chart review analysis of patients with cancer who received Nivolumab or Pembrolizumab, from 2015 till early 2019, at our institution. Among the patients who received immunotherapy, we identified those with underlying autoimmune conditions, excluding thyroid disease, and then developed a disease flare.
Results: Out of 419 patients, 121 received Pembrolizumab and 298 received Nivolumab. Eighteen patients in Pembrolizumab group and 21 in Nivolumab group had underlying autoimmune conditions. The most common autoimmune conditions were rheumatoid arthritis (RA), psoriatic arthritis (PA), sarcoidosis and inflammatory bowel disease (IBD). The most common treated malignancies were lung (53%) followed by melanoma (20%).
Out of the 39 patients, 11 (28%) (4 from Pembrolizumab and 7 from Nivolumab) developed disease flare. Three had RA, 2 PA, 2 lichen planus, 2 IBD, one multiple sclerosis and one polymyalgia rheumatica flares respectively.
Treatment was discontinued for 4 patients, continued without interruption for 4 patients, held for 2 patients and dose reduced for one. The majority (81%) developed a flare within first 3 cycles of initiation of treatment.
Conclusions: In our study population, patients with underlying autoimmune conditions tolerated immunotherapy fairly well. Treatment was stopped in only 4 out of 39 (10%) patients. We believe that further research is warranted in this arena.
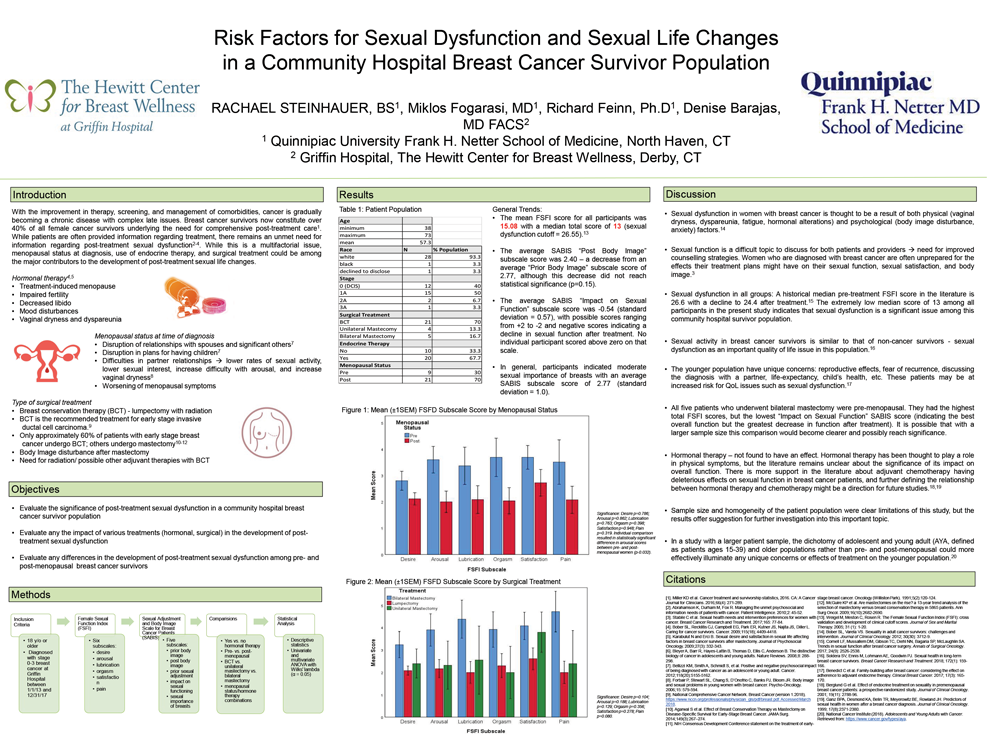
Risk Factors for Sexual Dysfunction and Sexual Life Changes in a Community Hospital Breast Cancer Survivor Population
Rachael Steinhauer, B.S1, Miklos Fogarasi M.D.1, Richard Feinn, Ph.D.1, Denise Barajas, M.D.2
1 Frank H. Netter, MD School of Medicine, 2 Griffin Hospital Department of Breast SurgeryBackground: Breast cancer survivors now constitute over 40% of all female cancer survivors. Addressing post-treatment sexual dysfunction, body image disturbance, and decreased sexual satisfaction is of particular importance to this population. Menopausal status, use of endocrine therapy, and type of surgical treatment could each play a role in the development of these survivorship issues, but their roles individually and in combination are less explored.
Methods: Thirty breast cancer survivors were surveyed using the Sexual Adjustment and Body Image Scale (SABIS) and the Female Sexual Function Index (FSFI). This study explores the previously stated factors as they relate to sexual health outcomes in a community hospital breast cancer survivor population using multivariate and univariate linear regression analysis.
Results: Sexual dysfunction was encountered in all groups. The median total FSFI score across all participants was 13 (sexual dysfunction cutoff = 26.55). Negative average scores for the SABIS “Impact on Sexual Functioning” subscale indicated a consistent mild decline in sexual function after all treatment. The FSFI “Arousal” scores in post-menopausal women were significantly lower than in pre-menopausal women (p=0.032). Treatment-specific analysis was limited by small sample size and statistical comparisons did not yield significance.
Conclusions: The results of this pilot study indicate that evaluating sexual health following breast cancer treatment is an important aspect of survivorship care in a community hospital population. In order to more specifically address this issue, it will be important to further explore factors that might lead to worsening of sexual function, particularly focusing on pre-menopausal patients.
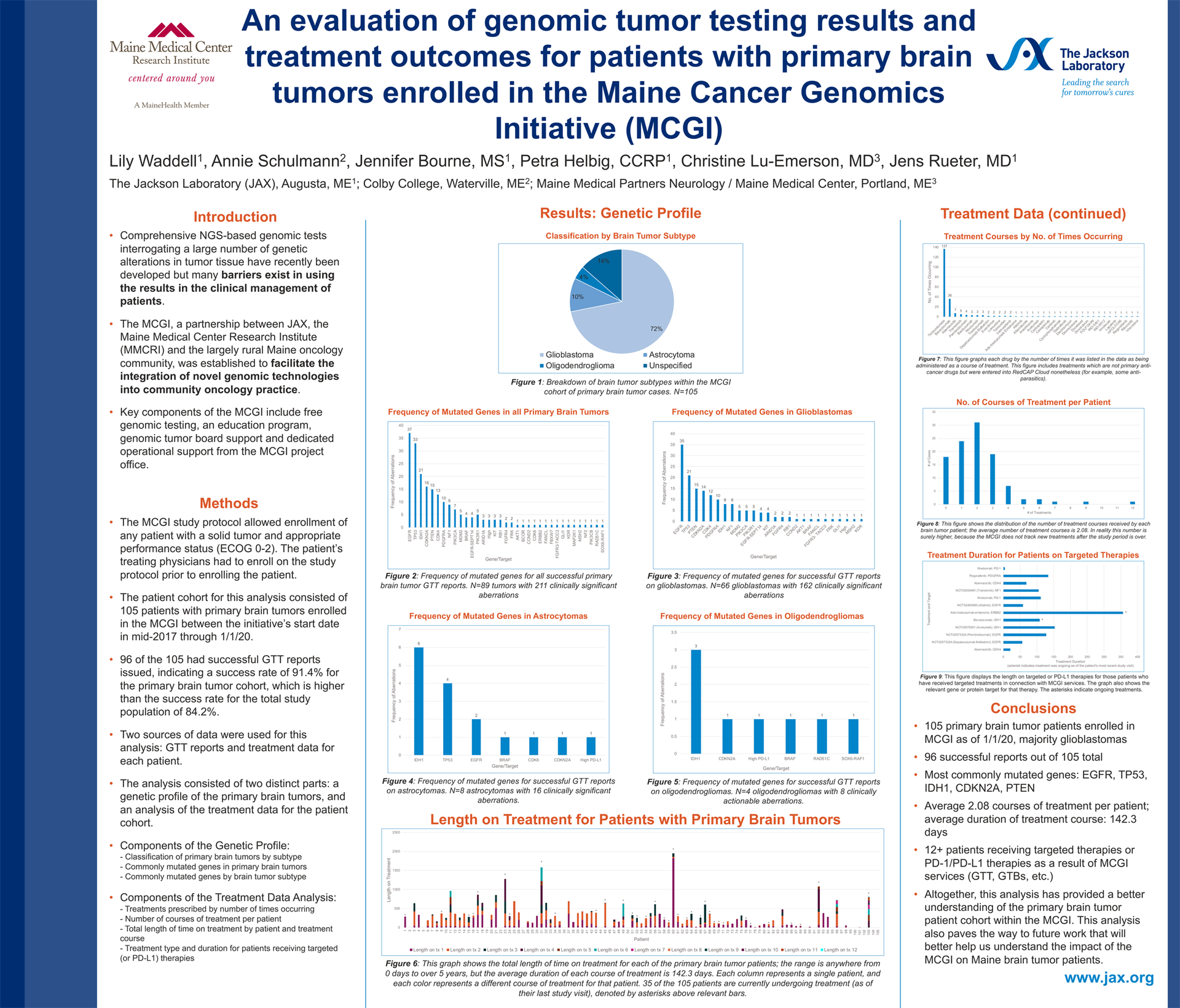
An evaluation of genomic tumor testing results and treatment outcomes for patients with primary brain tumors enrolled in the Maine Cancer Genomics Initiative (MCGI)
Lily Waddell1, Annie Schulmann2, Jennifer Bourne, MS1, Petra Helbig, CCRP1, Christine Lu-Emerson, MD3, Jens Rueter, MD1
1 The Jackson Laboratory (JAX), 2 Colby College, 3 Maine Medical Partners Neurology / Maine Medical Center
Background: The Maine Cancer Genomics Initiative (MCGI), a statewide collaboration between JAX and community and academic oncology practices in Maine, provides cancer patients and oncologists access to genomic tumor testing (GTT) along with education and decision support through genomic tumor boards. The Initiative’s overall aim is to overcome implementation barriers for precision oncology and to understand GTT outcomes.
Methods: Data from 105 patients with primary brain tumors enrolled in MCGI were evaluated to characterize the genomic tumor testing results and treatment outcomes for these patients. Cases were examined by brain tumor subtype and clinically actionable gene aberrations. Additionally, treatment data was examined to determine which treatments patients received and for how long.
Results: The 105 primary brain tumors consisted of 74 glioblastomas, 13 astrocytomas, 5 oligodendrogliomas, and 13 unspecified or other subtypes. 96/105 patients received successful GTT results. A total of 208 clinically actionable gene aberrations were listed in the successful reports. The most commonly mutated genes were, in order: EGFR, TP53, IDH1, PTEN, and CDKN2A. The average number of courses of treatment administered to each patient was 2.08; average duration of each course of treatment was 142.3 days. The most commonly prescribed drug was temozolomide, followed by bevacizumab, abemaciclib, trametinib, and pembrolizumab.
Conclusions: Altogether, this analysis provides both genetic and treatment profiles for patients with primary brain tumors who participate in the MCGI. The data collected and examined in this study will help better understand the impact of MCGI and GTT on Maine brain tumor patients.